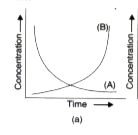
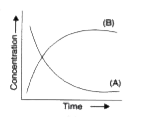
Consider the reaction A→B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figure correctly describes the change in concentration of reactants and products with time ?
A
B
C
D
Video Solution
Text Solution
The correct Answer is:B
With passage of time, concentration of [A] decreases expontially and that of [B] increases expontially.
|
Topper's Solved these Questions
- CHEMICAL KINETICSBook:ICSEChapter:CHEMICAL KINETICSExercise:EXERCISE (PART- I (OBJECTIVE QUESRTIONS) C. CORRECT THE FOLLOWING STATEMENTS BY CHANGING THE UNDERLINED PART OF THE STATEMENT ( DO NOT CHANGE THE WHOLE SENTENCE .))
Explore 39 Videos
- CHEMICAL KINETICSBook:ICSEChapter:CHEMICAL KINETICSExercise:EXERCISE (PART- I (OBJECTIVE QUESRTIONS) D. MATCH THE FOLLOWING )
Explore 3 Videos
- CHEMICAL KINETICSBook:ICSEChapter:CHEMICAL KINETICSExercise:EXERCISE (PART- I (OBJECTIVE QUESRTIONS)A.FILL IN THE BLANKS)
Explore 54 Videos
- BIOMOLECULESBook:ICSEChapter:BIOMOLECULESExercise:ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)
Explore 21 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:ICSEChapter:CHEMISTRY IN EVERYDAY LIFEExercise:EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))
Explore 19 Videos
Similar Questions
Knowledge Check
Consider the reaction A→B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figure correctly describes the change in concentration of reactants and products with time ?
A B
B C
C D
D
Consider the reaction A→B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figure correctly describes the change in concentration of reactants and products with time ?
A B
B C
C D
D
Consider the reaction A→B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figure correctly describes the change in concentration of reactants and products with time ?
A B
B C
C D
D
An increse in the concentration of the reactants of a reaction leads to change in:
View SolutionAssertion (A) : For a first order, the concentration of a reaction decreases exponentially with time.
Reason (R ): The rate of reaction at any time depends upon the concentration of the reactant at that time.
View SolutionIf concentration of reactant is increased by 2 times then K
becomes :-
View SolutionWhich of the following expresison is correct for first order reaction (c0 refers to initial concentration of reactant)
View SolutionThe configurations of the reactant and the product in the following reaction, respectively, are
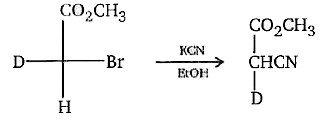
View Solution
