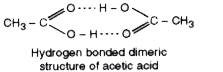Acetic acid exists as a dimer in benzene. Write the structure of this dimer.
টপার এই প্রশ্নগুলো সমাধান করেছে
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:EXERCISE (PART-II DESCRIPTIVE QUESTIONS) ( SHORT QUESTIONS)
Explore 581 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ISC EXAMINATION QUESTIONS (PART-I OBJECTIVE QUESTIONS) (FILL IN THE BLANKS)
Explore 27 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:EXERCISE (PART-I OBJECTIVE QUESTIONS)
Explore 303 Videos
- ALCOHOLS, PHENOLS AND ETHERS Book:ICSEChapter:ALCOHOLS, PHENOLS AND ETHERS Exercise:ISC EXAMINATION QUESTIONS
Explore 25 Videos
- APPENDIX II ( TYPICAL CONVERSIONS)Book:ICSEChapter:APPENDIX II ( TYPICAL CONVERSIONS)Exercise:AROMATIC CONVERSIONS
Explore 98 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Acetic acid exists as dimer in benzene due to
Aa condensation reactionBhydrogen bonding.Cpresence of carbonyl groupDpresence of hydrogen atom at α-carbon atom.Acetic acid exists as dimer in benzene due to
Aa condensation reactionBhydrogen bonding.Cpresence of carbonyl groupDpresence of hydrogen atom at α-carbon atom.Acetic acid exists as dimer in benzene due to
Aa condensation reactionBhydrogen bonding.Cpresence of carbonyl groupDpresence of hydrogen atom at α-carbon atom.
Read the following paragraph and answer the question given below:
Freezing point of benzene is 278.4K and heat of fusion of benzene is 10.042 KJ/mol. Acetic acid exists partly as dimer in benzene solution. The freezing point of 0.02 mol fraction of acetic acid in benzene is 277.4 K.
The degree of dimerisation of acetic acid in benzene is
View SolutionRead the following paragraph and answer the question given below:
Freezing point of benzene is 278.4K and heat of fusion of benzene is 10.042 KJ/mol. Acetic acid exists partly as dimer in benzene solution. The freezing point of 0.02 mol fraction of acetic acid in benzene is 277.4 K.
The equilibrium constant for dimerisation of acetic in benzene is
View SolutionRead the following paragraph and answer the question given below:
Freezing point of benzene is 278.4K and heat of fusion of benzene is 10.042 KJ/mol. Acetic acid exists partly as dimer in benzene solution. The freezing point of 0.02 mol fraction of acetic acid in benzene is 277.4 K.
The molal cryoscopic constant of benzene in Kmolality−1 is
View SolutionAcetic acid dimerises in benzene solution.The van't Hoff factor for the dimerisation of acetic acid is 0.8. The % of dimerisation of acetic acid is
View SolutionThe freezing point of 0.02 mole fraction acetic acid in benzene is 277.4K. Acetic acid exists partly as dimer. Calculate the equilibrium constant for dimerization. The freezing point of benzene is 278.4K and the heat the fusion of benzene is 10.042kJmol−1. Assume molarity and molality same.
View Solution