Write the structures of the main products of the following reactions :
H3C−C≡C−HHg2+,H2SO4−−−−−−−−→
To solve the given reaction of propyne (CH₃C≡CH) with Hg²⁺ in the presence of sulfuric acid (H₂SO₄), we will follow these steps:
Step 1: Identify the Reactants
The reactant is propyne, which has the structure:
- Propyne (CH₃C≡CH)
Step 2: Understand the Reaction Conditions
The reaction is carried out in the presence of mercury(II) ions (Hg²⁺) and sulfuric acid (H₂SO₄). This indicates that we are likely performing an electrophilic addition reaction.
Step 3: Electrophilic Addition of Hg²⁺
In the presence of Hg²⁺ and H₂SO₄, the triple bond in propyne will undergo electrophilic addition. The mercury ion will add to one of the carbon atoms of the triple bond, forming a vinyl cation.
Step 4: Formation of the Vinyl Cation
The addition of Hg²⁺ to propyne results in the formation of a vinyl cation:
- Vinyl cation (CH₃C^+=CH₂)
Step 5: Nucleophilic Attack by Water
The vinyl cation will then undergo nucleophilic attack by water (from the sulfuric acid), leading to the formation of an enol:
- Enol (CH₃C(OH)=CH₂)
Step 6: Tautomerization to Ketone
The enol formed will undergo tautomerization to yield a ketone. The enol (CH₃C(OH)=CH₂) will rearrange to form:
- Ketone (CH₃C(=O)CH₃), which is acetone.
Final Structures of the Main Products
1. Enol: CH₃C(OH)=CH₂ (Prop-1-en-2-ol)
2. Ketone: CH₃C(=O)CH₃ (Acetone)
Summary of the Reaction
The main products of the reaction of propyne with Hg²⁺ and H₂SO₄ are:
- Enol: Prop-1-en-2-ol
- Ketone: Acetone
The reactant is propyne, which has the structure:
- Propyne (CH₃C≡CH)
The reaction is carried out in the presence of mercury(II) ions (Hg²⁺) and sulfuric acid (H₂SO₄). This indicates that we are likely performing an electrophilic addition reaction.
In the presence of Hg²⁺ and H₂SO₄, the triple bond in propyne will undergo electrophilic addition. The mercury ion will add to one of the carbon atoms of the triple bond, forming a vinyl cation.
The addition of Hg²⁺ to propyne results in the formation of a vinyl cation:
- Vinyl cation (CH₃C^+=CH₂)
The vinyl cation will then undergo nucleophilic attack by water (from the sulfuric acid), leading to the formation of an enol:
- Enol (CH₃C(OH)=CH₂)
The enol formed will undergo tautomerization to yield a ketone. The enol (CH₃C(OH)=CH₂) will rearrange to form:
- Ketone (CH₃C(=O)CH₃), which is acetone.
1. Enol: CH₃C(OH)=CH₂ (Prop-1-en-2-ol)
2. Ketone: CH₃C(=O)CH₃ (Acetone)
The main products of the reaction of propyne with Hg²⁺ and H₂SO₄ are:
- Enol: Prop-1-en-2-ol
- Ketone: Acetone
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ISC EXAMINATION QUESTIONS (PART-I OBJECTIVE QUESTIONS) (FILL IN THE BLANKS)
Explore 27 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ISC EXAMINATION QUESTIONS (PART-I OBJECTIVE QUESTIONS) (MULTIPLE CHOICE QUESTION)
Explore 24 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:EXERCISE (PART-II DESCRIPTIVE QUESTIONS) (VERY SHORT QUESTIONS)
Explore 282 Videos
- ALCOHOLS, PHENOLS AND ETHERS Book:ICSEChapter:ALCOHOLS, PHENOLS AND ETHERS Exercise:ISC EXAMINATION QUESTIONS
Explore 25 Videos
- APPENDIX II ( TYPICAL CONVERSIONS)Book:ICSEChapter:APPENDIX II ( TYPICAL CONVERSIONS)Exercise:AROMATIC CONVERSIONS
Explore 98 Videos
Similar Questions
Write the structures of the main products in the following reactions :
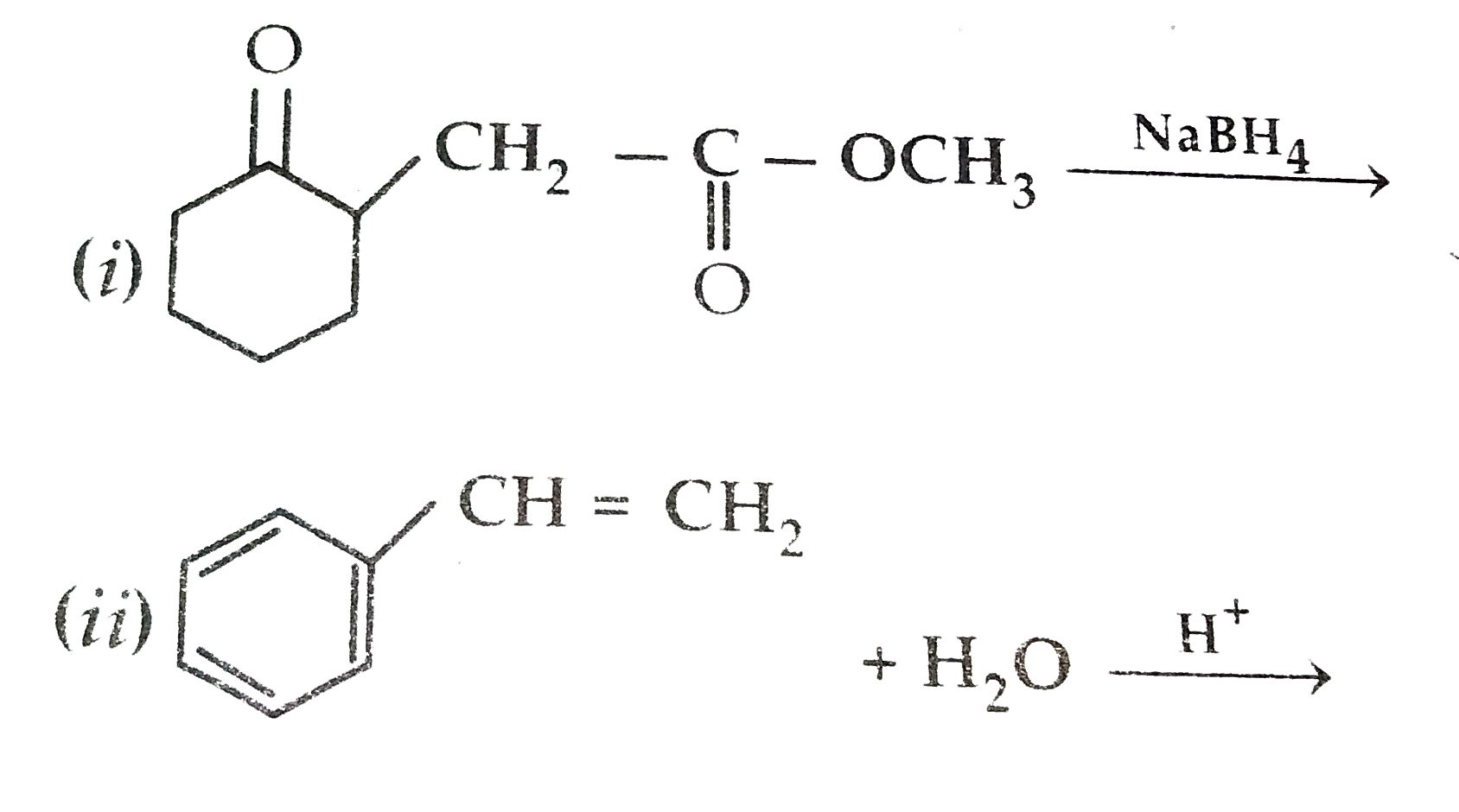
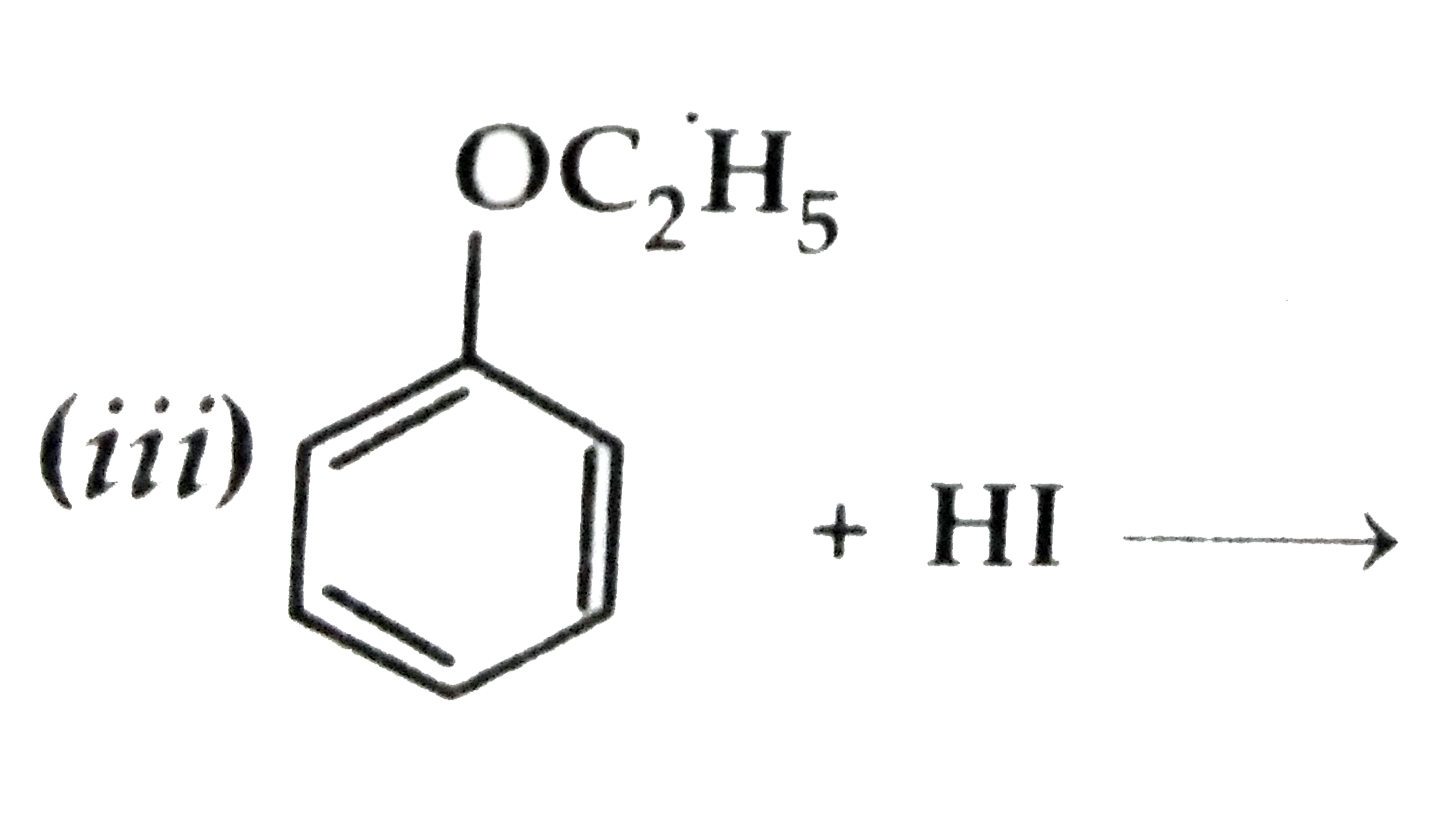
View Solution(a) Give chemical tests to distinguish between
(i) Phenol and Benzoic acid
(ii) Benzophenone and Acetophenone
(b) Write the strucutre of the main products of following reactions :
(i)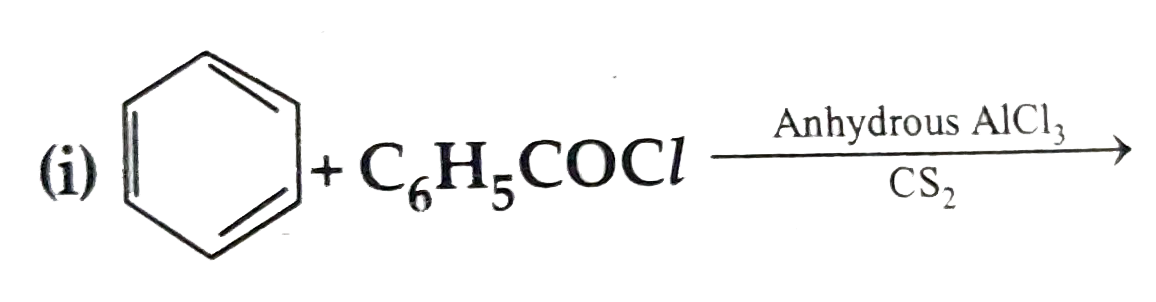
(ii) H3C−C≡C−HHg2+,H2SO4−−−−−−−−→
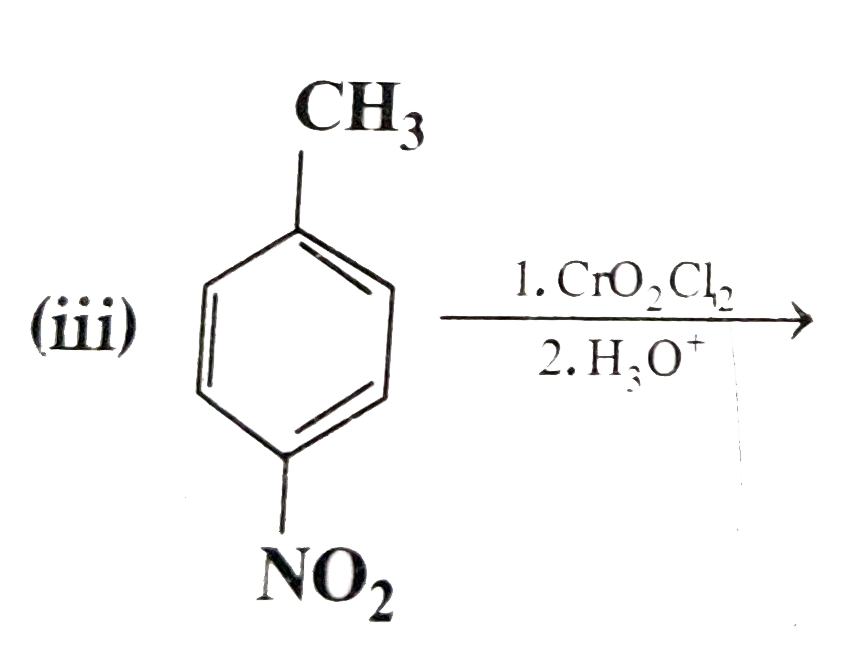 .
.
View Solution
Knowledge Check
Predict product of following reaction,
H3C−O∣∣C−O−C2H5NaBH4−−−−→ACH3−CH2−OHBNo reactionCH3C−O∣∣C−OHDH2C=CH2
Write structures of the products of the following reactions:
(i) CH3−CH=CH2H2O/H+−−−−−→
(ii)
(iii) CH3−CH2−C | CH3H−CHONaBH4−−−−→
View SolutionThe main product of the following reaction is C6H5CH2(OH)CH(CH3)2Conc.H2SO4−−−−−−−→?
View SolutionDraw the stereochemical structures of the products in the following reactions.
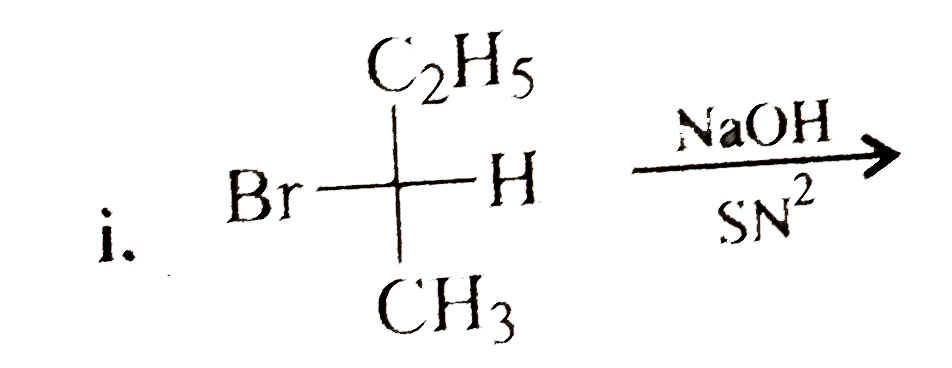 .
.
R−C≡C−RH2,Lindlar catalyst−−−−−−−−−−−→
View SolutionThe product of following reaction is:
_2019_CBT4_E01_007_Q01.png) H2SO4(Cat.)−−−−−−−−→CHCl3
H2SO4(Cat.)−−−−−−−−→CHCl3
View SolutionWrite the main products of the following reactions:
(i) C6H5N+2Cl−H3PO2+H2O−−−−−−−−→?
(ii)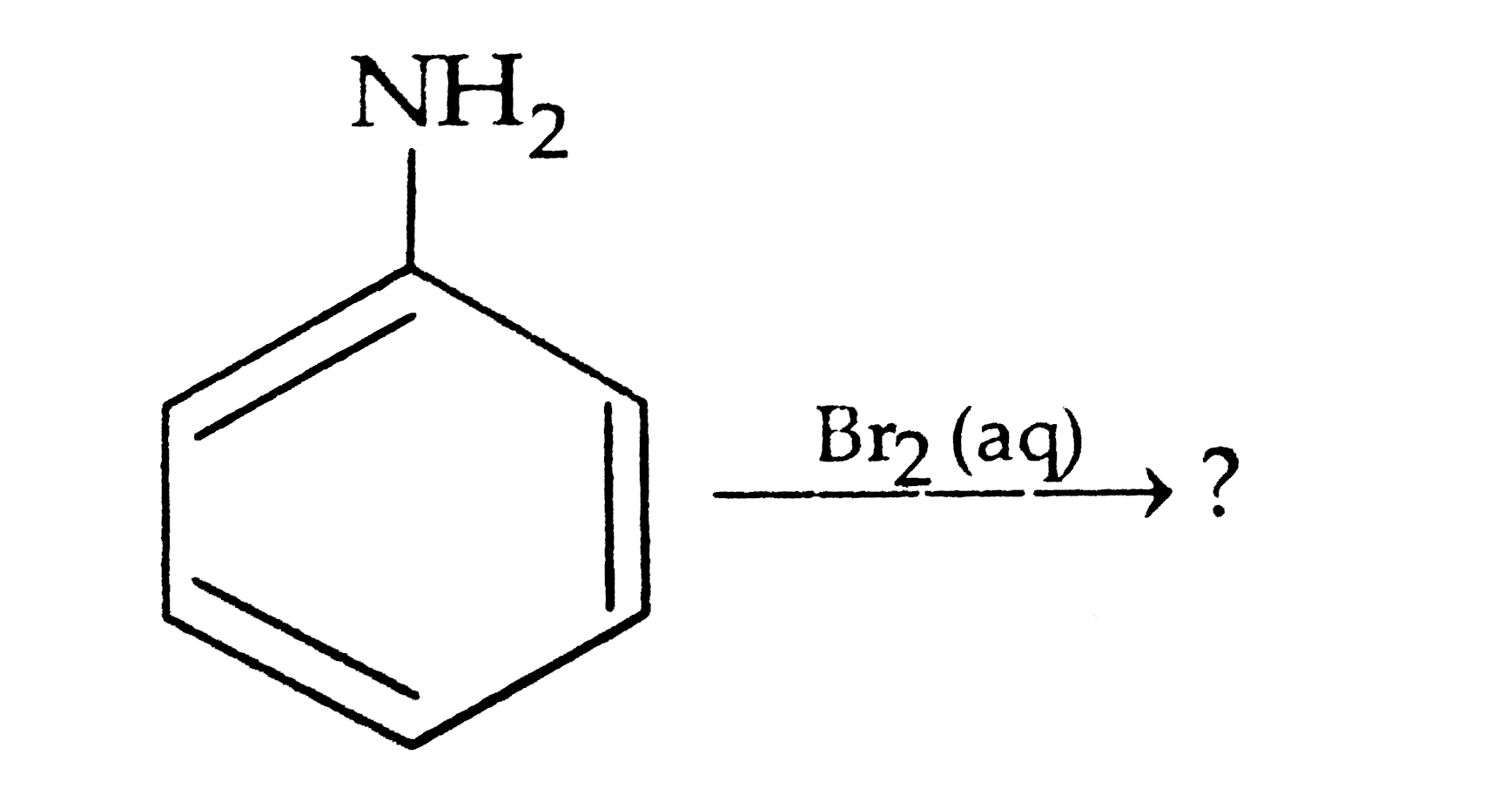
(iii) CH3−C∣∣O−NH2Br2+NaOH−−−−−−−→?
View SolutionWrite the main products of the following reactions:
(i) C6H5N+2Cl−H3PO2+H2O−−−−−−−−→?

(iii) CH3−C∣∣O−NH2Br2+NaOH−−−−−−−→?
View SolutionFill in the blanks with appropriate structure of reaction products in the following trasformation:
HOOC−C6H4−CH2−C6H5SOCl2−−−→Aanlydrous−−−−−→AlCl3BZn−Hg−−−−→HClC
View Solution
