Cyclopropane rearranges to form propene
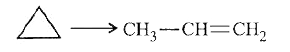
This follows first order kinetics. The rate constant is 2.714×10−3 sec−1. The initial concentration of cyclopropane is 0.29 M. What will be the concentration of cyclopropane after 100 sec?
0.035 M
0.22 M
0.145 M
0.0018 M
The correct Answer is:B
k=2.303tlog(a)(a−x)
(a-x) is the concentration left after 100 sec.
2.714×10−3=2.303100log(0.29)(a−x)
⇒0.27142.303=log(0.29)(a−x)⇒0.118=log(0.29)(a−x)
⇒(a−x)=0.22 M
Topper's Solved these Questions
- CHEMICAL KINETICSBook:MTG GUIDEChapter:CHEMICAL KINETICSExercise:CHECK YOUR NEET VITALS
Explore 25 Videos
- CHEMICAL KINETICSBook:MTG GUIDEChapter:CHEMICAL KINETICSExercise:AIPMT/NEET (MCQs)
Explore 22 Videos
- CHEMICAL KINETICSBook:MTG GUIDEChapter:CHEMICAL KINETICSExercise:AIPMT/NEET (MCQs)
Explore 22 Videos
- BIOMOLECULESBook:MTG GUIDEChapter:BIOMOLECULESExercise:AIPMT/NEET (MCQS)
Explore 19 Videos
- CHEMISTRY IN EVERYDAY LIFE Book:MTG GUIDEChapter:CHEMISTRY IN EVERYDAY LIFE Exercise:AIPMT/NEET
Explore 9 Videos
Similar Questions
Cyclopropane rearranges to form propene
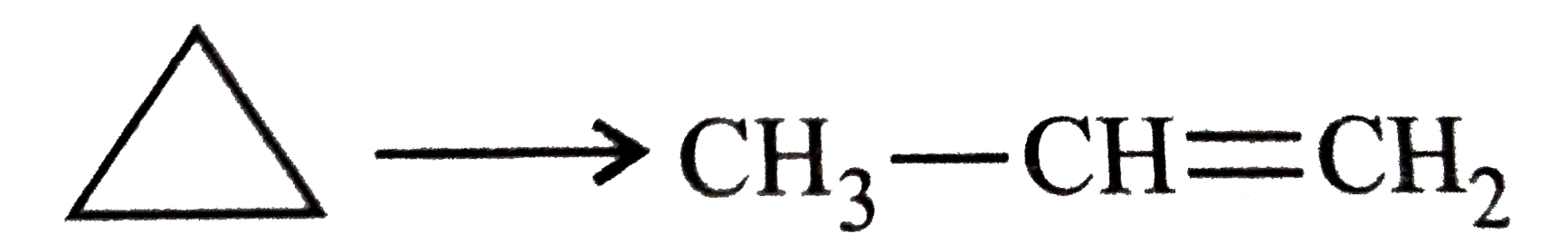 →CH3−CH=CH2
→CH3−CH=CH2
This follows first order kinetics. The rate constant is 2.714×10−3sec−1. The unitial concentration of cyclopropane is 0.29 M. What will be the concentraion of cyclopropane after 100 sec?
View SolutionThe rate constant of a first order reaction is 3×10−6 per second. If the initial concentration is 0.10M, the initial rate of reaction is
View Solution
Knowledge Check
Cyclopropane rearranges to form propane :
Δ→CH3−CH=CH2
This follows first order kinetics. The rate constant is 2.174×10−3sec−1 . The initial concentration of cyclopropane is 0.29M . What will be the concentration of cyclopropane after 100 secA0.035 MB0.22 MC0.145 MD0.0018 MCyclopropane rearranges to form propane :
Δ→CH3−CH=CH2
This follows first order kinetics. The rate constant is 2.174×10−3sec−1 . The initial concentration of cyclopropane is 0.29M . What will be the concentration of cyclopropane after 100 secA0.035MB0.22MC0.145MD0.0018MThe rate constant of a first order reaction is 3×10−5sec−1M If initial concentration is 0.10M, the initial rate is (Ms−1)
A3×10−6B3×10−5C3×10−8D3×10−7
The specific rate constant for a first order reaction is 60×10−4sec−1. If the initial concentration of the reactant is 0.01molL−1, the rate is
View SolutionFor the zero order reaction :
A → P, K=10−2(mol/litre)sec−1
If initial concentration of A is 0.3 M, then find concentration of A left after 10 sec.
View SolutionThe rate constant of a first order reaction is 3×10−6 per second. If the initial concentration is 0.10M, the initial rate of reaction is
View SolutionThe rate constant of a first order reaction is 3×10−6 per second. If the initial concentration is 0.10M, the initial rate of reaction is
View SolutionThe specific rate constant for a first order reaction is 60×10−4sec−1. If the initial concentration of the reactant is 0.01molL−1, the rate is
View Solution

