A solid AB has rock salt structure. How many atoms of A and B are present in the unit cell ?
To determine how many atoms of A and B are present in the unit cell of a solid AB with a rock salt structure, we can follow these steps:
1. Identify the Structure:
The rock salt structure is similar to that of NaCl, where A represents cations (A⁺) and B represents anions (B⁻). In this structure, the B⁻ ions form a face-centered cubic (FCC) arrangement.
2. Count the Atoms of B:
- In a face-centered cubic structure, there are 8 corner atoms and 6 face-centered atoms.
- Each corner atom contributes 18 of an atom to the unit cell, and each face-centered atom contributes 12 of an atom.
- Therefore, the total contribution from the corner atoms is:
8 corners×18=1 atom
- The total contribution from the face-centered atoms is:
6 faces×12=3 atoms
- Thus, the total number of B atoms in the unit cell is:
1+3=4 atoms of B
3. Count the Atoms of A:
- In the rock salt structure, the cations (A⁺) occupy the octahedral voids. The number of octahedral voids in a unit cell is equal to the number of atoms in the FCC lattice, which is Z=4.
- Therefore, the number of A atoms in the unit cell is:
4 atoms of A
4. Conclusion:
- In the unit cell of the solid AB with rock salt structure, there are 4 atoms of A and 4 atoms of B.
Final Answer:
- Number of A atoms: 4
- Number of B atoms: 4
---
The rock salt structure is similar to that of NaCl, where A represents cations (A⁺) and B represents anions (B⁻). In this structure, the B⁻ ions form a face-centered cubic (FCC) arrangement.
- In a face-centered cubic structure, there are 8 corner atoms and 6 face-centered atoms.
- Each corner atom contributes 18 of an atom to the unit cell, and each face-centered atom contributes 12 of an atom.
- Therefore, the total contribution from the corner atoms is:
8 corners×18=1 atom
- The total contribution from the face-centered atoms is:
6 faces×12=3 atoms
- Thus, the total number of B atoms in the unit cell is:
1+3=4 atoms of B
- In the rock salt structure, the cations (A⁺) occupy the octahedral voids. The number of octahedral voids in a unit cell is equal to the number of atoms in the FCC lattice, which is Z=4.
- Therefore, the number of A atoms in the unit cell is:
4 atoms of A
- In the unit cell of the solid AB with rock salt structure, there are 4 atoms of A and 4 atoms of B.
- Number of A atoms: 4
- Number of B atoms: 4
Similar Questions
An element occurs in bcc structure. How many atoms are present in its unit cell ?
View SolutionHow many O - atoms are present in Equanil.
View SolutionHow many types of H atoms are present in isobutane?
View SolutionHow many carbon atoms are present in one unit cell of diamond ?
View Solution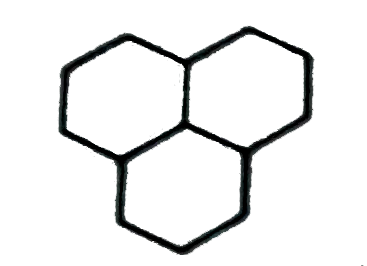
How many hydrogen atoms are present in the given structure ?
View SolutionHow many number of atoms are completely inside the HCP unit cell ?
View SolutionHow many number of atoms present in half of HCP unit cell
View SolutionHow many N atom are present in final product.
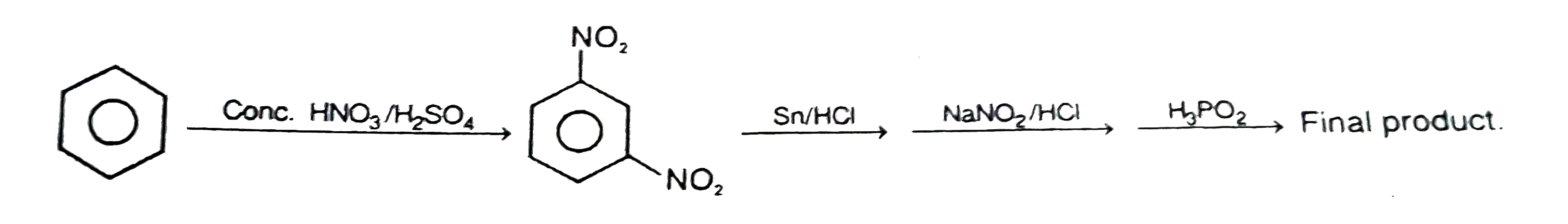
View SolutionA solid has a structure in which A atoms are located at the cube corners of the unit cell, B atoms are located at the cube edges of unit cell and the C atoms at the body centre. Formula of the compound
View SolutionHow many carbon atoms are present in a pentose sugar?
View Solution

