How does the density of crystal change due to Schottky defects ?
The density of crystals decreases because certain ions are missing from the lattice.
समान प्रश्न
ज्ञान जांच
In the Schottky defect:
Aan ion moves to interstitial position between the lattice pointsBelectrons are trapped in a lattice siteCsome lattice sites are vacantDsome extra cations are present in interstitial spacesExamine the given defected crystal
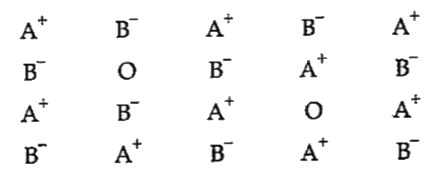
Answer the following questions:
How is the density of crystal affected by this defect?ASchottky defect, decreases, NaCl and KCl etcBFrenkel defect, increases, As and Si etcCSchottky defect, increases, As and Bi etcDFrenkel defect, decreases, NaCl, KCl etc
Defects which do not disturb the stoichiometry are called stochiometric defect. At the time of crystal formation an ion may not occupy its expected position leaving a void or hole at that site. As a metter of fact one positive and one negative ion are missing from their respective positions leaving behind a pair of holes. This vacancy is a Schottky defect. Due to this defect density decreases. Defect arises in which certain atoms or ions have migrated to interstitial position some distance removed from the holes which they vacated. In this case there is no change in the density of the crystal.
In the Schottky defect
View SolutionFrenkel defect does not change the density of the ionic crystal whereas, Schottky defect lowers the density of ionic crystal. Give a reason.
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?
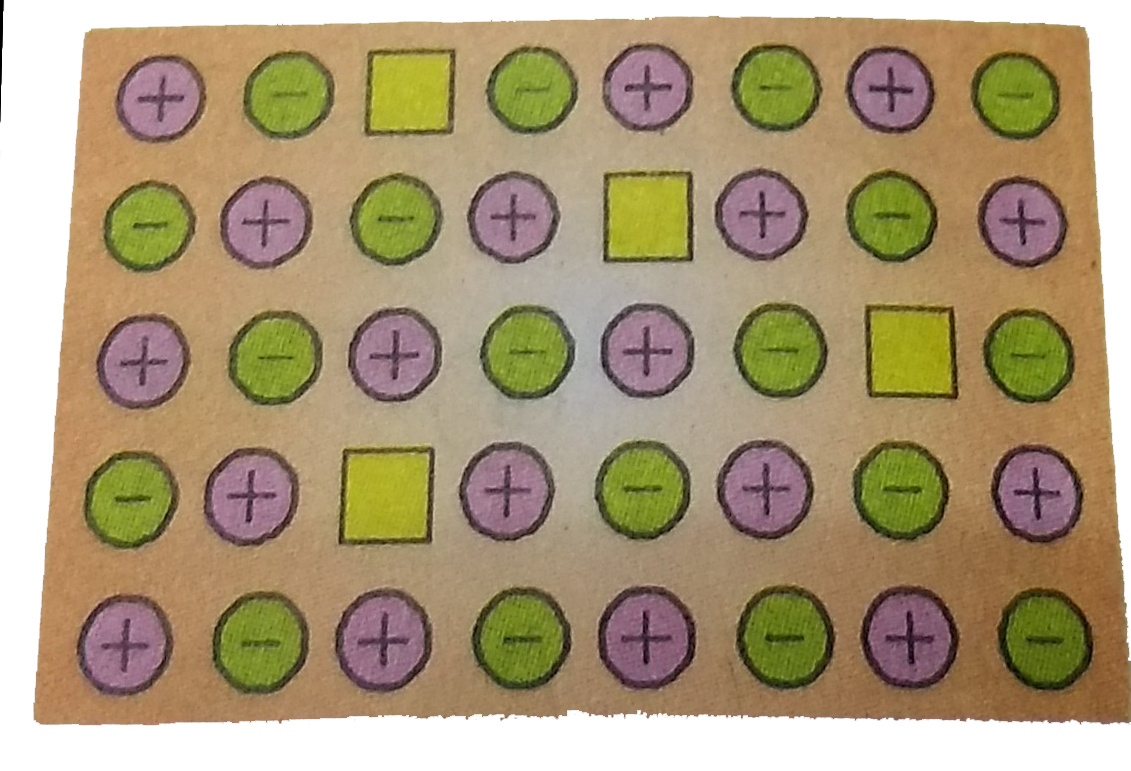
View SolutionHow Schottky and Frenkel defects occur in ionic crystals? How does the density of a crystal gets affected by the presence of these defects ?
View SolutionExamiine the given defective crystal

(i) What type of stoichiometric defect is shown by the the crystal ?
(ii) How is the density of the crystal affected by this defect ?
(iii) What type of ionic substance show such defect ?
View SolutionWhich of the following statement for crystals having Schottky defect is not correct?
View Solution
