A and B are functional isomers of compound C3H6O. On heating with NaOH and I2 isomer A froms yellow percipitate of iodoform whereas isomer B does not form any precipitate. Write the formulae of A and B.
To solve the problem, we need to identify the functional isomers A and B of the compound with the molecular formula C3H6O. We know that isomer A gives a positive iodoform test, while isomer B does not.
1. Identify the Molecular Formula:
The molecular formula given is C3H6O. This indicates that the compound could be either an aldehyde or a ketone.
2. Understanding the Iodoform Test:
The iodoform test is a qualitative test for the presence of a methyl ketone or a compound that can be oxidized to a methyl ketone. A compound must have the structure RCOCH3 (where R can be any alkyl group) to give a positive iodoform test.
3. Determine Isomer A:
Since isomer A gives a positive iodoform test, it must contain a methyl ketone group. The only possible structure for isomer A that fits the C3H6O formula and has a methyl ketone group is acetone (or propan-2-one), which has the structure:
CH3COCH3
4. Determine Isomer B:
Isomer B does not give a positive iodoform test, which means it cannot have a methyl ketone group. The only other possible structure for C3H6O that is an aldehyde is propanal (or propanal), which has the structure:
CH3CH2CHO
5. Final Identification:
- Isomer A (which gives a positive iodoform test) is acetone: CH3COCH3.
- Isomer B (which does not give a positive iodoform test) is propanal: CH3CH2CHO.
Conclusion:
The formulae of the isomers are:
- Isomer A: Acetone (CH3COCH3)
- Isomer B: Propanal (CH3CH2CHO)
The molecular formula given is C3H6O. This indicates that the compound could be either an aldehyde or a ketone.
The iodoform test is a qualitative test for the presence of a methyl ketone or a compound that can be oxidized to a methyl ketone. A compound must have the structure RCOCH3 (where R can be any alkyl group) to give a positive iodoform test.
Since isomer A gives a positive iodoform test, it must contain a methyl ketone group. The only possible structure for isomer A that fits the C3H6O formula and has a methyl ketone group is acetone (or propan-2-one), which has the structure:
CH3COCH3
Isomer B does not give a positive iodoform test, which means it cannot have a methyl ketone group. The only other possible structure for C3H6O that is an aldehyde is propanal (or propanal), which has the structure:
CH3CH2CHO
- Isomer A (which gives a positive iodoform test) is acetone: CH3COCH3.
- Isomer B (which does not give a positive iodoform test) is propanal: CH3CH2CHO.
The formulae of the isomers are:
- Isomer A: Acetone (CH3COCH3)
- Isomer B: Propanal (CH3CH2CHO)
Similar Questions
(a) Write the chemical reaction involved in Wolf-Kishner reduction.
(b) Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction :
C6H5COCH3,CH3−CHO,CH3COCH3
(c) Why carboxylic acid does not give reactions of carbonyl group ?
(d) Write the product in the following reaction.
CH3CH2CH=CH−CH2CN1.(i−Bu)2AlH−−−−−−−−−−→2.H2O
(e) A and B are two functional isomers of compound C3H6O. On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
View SolutionWrite chain and functional isomers of C4H8O.
View Solution
Knowledge Check
An aromatic compound A of the molecular formula C8H10O on reaction with iodine and dilute NaOH gives a yellow precipitate. The structure of the compound is expected to be :
A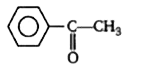 BC6H5CHOHCH3C
BC6H5CHOHCH3C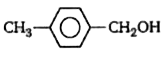 D
D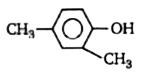
Draw the functional isomer of C3H6O .
View SolutionWrite the isomers of the compound having formula C4H9Br.
View SolutionWrite the possible isomers of the C6H4(OH)2
View SolutionWrite the possible isomers of the C6H4(CH3)2
View Solution(A), (B) and (C ) are three non-cylic funtional isomers of a carbonyl compound with molecular formula C4H8O. Isomers (A) and (C ) give positive Tollen's test whereas isomer (B) does not give Tollens' test but gives positive iodoform test. Isomers (A) and (B) on reduction with Zn(Mg) | conc. HCl give the same product (D).
(a) Write the structures of (A), (B) ,(C) and (D).
(b) Out of (A), (B) and (C ) isomers, which one is least reactive towards addition of HCN ?
View SolutionWrite the possible functional isomers having the formula C3H6O2
View SolutionA metal complex having composition Cr(NH3)4CI2Br has been isolated in two forms A and B. The form A reacts with AgNO3 to give a white precipitate readily soluble in dilute aqueous ammonia whereas B gives a pale yellow precipitate soluble in concentrated ammonia.
(i) Write the formulae of isomers A and B.
(ii) State the hybridisation of chromium in each of them.
(iii) Calculate the magnetic moment (spin only) of the isomer A.
View Solution
