Write IUPAC name of CH3OCH3.
To determine the IUPAC name of the compound CH₃OCH₃, follow these steps:
Step 1: Identify the Structure
The given molecular formula CH₃OCH₃ indicates that there are two methyl groups (CH₃) connected by an oxygen atom. This structure suggests that it is an ether.
Step 2: Recognize the Functional Groups
In this compound, the functional group present is an ether, characterized by the presence of an oxygen atom bonded to two alkyl groups. In this case, both alkyl groups are methyl groups.
Step 3: Name the Alkyl Groups
The two alkyl groups present in the compound are both methyl groups (CH₃). In IUPAC nomenclature, when both groups are the same, we can use the prefix "di-" to indicate that there are two identical groups.
Step 4: Combine the Names
Since both alkyl groups are methyl, we can combine the names to form "dimethyl." However, because we have an ether, we also need to include the term "ether" to indicate the functional group.
Step 5: Write the Final IUPAC Name
The IUPAC name for the compound CH₃OCH₃ can be written as "dimethyl ether." However, in this case, we can also refer to it as "methoxy methane" since it has one carbon chain (methane) and a methoxy group (–OCH₃) attached to it.
Final Answer
The IUPAC name of the compound CH₃OCH₃ is methoxy methane.
---
The given molecular formula CH₃OCH₃ indicates that there are two methyl groups (CH₃) connected by an oxygen atom. This structure suggests that it is an ether.
In this compound, the functional group present is an ether, characterized by the presence of an oxygen atom bonded to two alkyl groups. In this case, both alkyl groups are methyl groups.
The two alkyl groups present in the compound are both methyl groups (CH₃). In IUPAC nomenclature, when both groups are the same, we can use the prefix "di-" to indicate that there are two identical groups.
Since both alkyl groups are methyl, we can combine the names to form "dimethyl." However, because we have an ether, we also need to include the term "ether" to indicate the functional group.
The IUPAC name for the compound CH₃OCH₃ can be written as "dimethyl ether." However, in this case, we can also refer to it as "methoxy methane" since it has one carbon chain (methane) and a methoxy group (–OCH₃) attached to it.
The IUPAC name of the compound CH₃OCH₃ is methoxy methane.
Similar Questions
Write the IUPAC names of (i) CH3OCH2CH2OH (ii) CH3OCH2OCH3 and (iii)
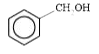
View SolutionWrite IUPAC name of the following
(h) CH3CH2OCH2CH3
View SolutionWrite IUPAC name of the following
(i) CH3OCH2CH2CH3
View SolutionWrite IUPAC name of the following
(h) CH3CH2OCH2CH3
View SolutionWrite IUPAC name of the following
(i) CH3OCH2CH2CH3
View SolutionWrite IUPAC name of the following
(i) CH3OCH2CH2CH3
View SolutionWrite the IUPAC names of: CH_(3)OCH_(2)CH_(2)OCH_(2)CH_(3)
View SolutionThe IUPAC name of compound
CH3OCH2CH2CH2OCH2CH3 is
View Solution

