In which one of following reactions of glycolysis, oxidation takes place
Glucose 6-P to fructose 6-P
Fructose 6-P to fructose 1,6-biphosphate
1,3-biphosphoglycerate to 3-phosphogl- yceric acid
3-phosphoglyceraldehyde to 1, 3-bipho-sphoglycerate
The correct Answer is:D
To determine in which reaction of glycolysis oxidation takes place, we can follow these steps:
1. Understand Glycolysis: Glycolysis is the metabolic pathway that converts glucose into pyruvate, producing ATP and NADH in the process. It occurs in the cytoplasm and consists of ten enzyme-catalyzed reactions.
2. Identify the Reactions: Review the reactions involved in glycolysis. The key reactions include:
- Glucose to Glucose-6-phosphate
- Glucose-6-phosphate to Fructose-6-phosphate
- Fructose-6-phosphate to Fructose-1,6-bisphosphate
- Fructose-1,6-bisphosphate to Glyceraldehyde-3-phosphate and Dihydroxyacetone phosphate
- Glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate
- 1,3-bisphosphoglycerate to 3-phosphoglycerate
- 3-phosphoglycerate to 2-phosphoglycerate
- 2-phosphoglycerate to Phosphoenolpyruvate
- Phosphoenolpyruvate to Pyruvate
3. Identify Oxidation Reaction: Oxidation involves the loss of electrons or hydrogen atoms. In glycolysis, the reaction where oxidation occurs is when Glyceraldehyde-3-phosphate (G3P) is converted to 1,3-bisphosphoglycerate (1,3-BPG). During this reaction, NAD+ is reduced to NADH, indicating that oxidation has taken place.
4. Select the Correct Option: Based on the analysis, the correct answer is the reaction:
- Glyceraldehyde-3-phosphate (G3P) → 1,3-bisphosphoglycerate (1,3-BPG)
This corresponds to option number four.
5. Conclusion: Therefore, the reaction in glycolysis where oxidation occurs is the conversion of Glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
Final Answer:
The reaction in glycolysis where oxidation takes place is the conversion of Glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate (Option 4).
---
- Glucose to Glucose-6-phosphate
- Glucose-6-phosphate to Fructose-6-phosphate
- Fructose-6-phosphate to Fructose-1,6-bisphosphate
- Fructose-1,6-bisphosphate to Glyceraldehyde-3-phosphate and Dihydroxyacetone phosphate
- Glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate
- 1,3-bisphosphoglycerate to 3-phosphoglycerate
- 3-phosphoglycerate to 2-phosphoglycerate
- 2-phosphoglycerate to Phosphoenolpyruvate
- Phosphoenolpyruvate to Pyruvate
- Glyceraldehyde-3-phosphate (G3P) → 1,3-bisphosphoglycerate (1,3-BPG)
This corresponds to option number four.
The reaction in glycolysis where oxidation takes place is the conversion of Glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate (Option 4).
Topper's Solved these Questions
- RESPIRATIONBook:DINESH PUBLICATION ENGLISHChapter:RESPIRATIONExercise:CHECK YOUR GRASP
Explore 20 Videos
- RESPIRATIONBook:DINESH PUBLICATION ENGLISHChapter:RESPIRATIONExercise:CHECK YOUR GRASP
Explore 20 Videos
- REPTILIA-CREEPING VERTEBRATESBook:DINESH PUBLICATION ENGLISHChapter:REPTILIA-CREEPING VERTEBRATESExercise:CHECK YOUR GRASP
Explore 10 Videos
- ROOTBook:DINESH PUBLICATION ENGLISHChapter:ROOTExercise:CHECK YOUR GRASP
Explore 10 Videos
Similar Questions
Knowledge Check
In Which of the following reaction an acid-base reaction takes place ?
A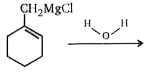 B
B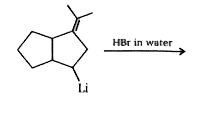 C
C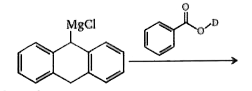 DAll of these
DAll of these
Which one of the following is wrong about glycolysis
View SolutionIn which of the following reaction of glycolysis, a molecule of water is removed from the substrate
View SolutionWhich of the following reaction is oxidation- reduction?
View SolutionConsider the following reactions :
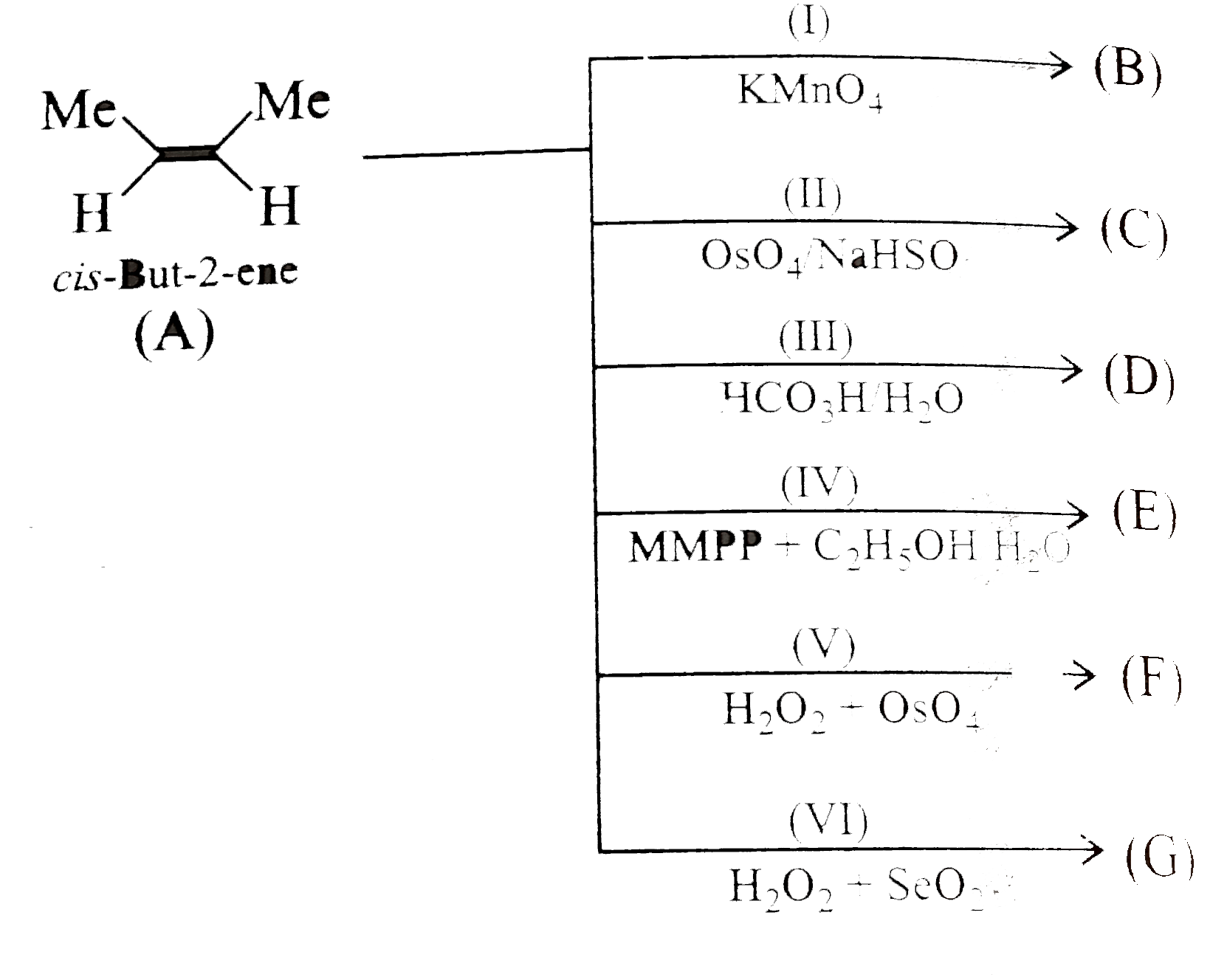
Syn-addition takes place in :
View SolutionConsider the following reactions :
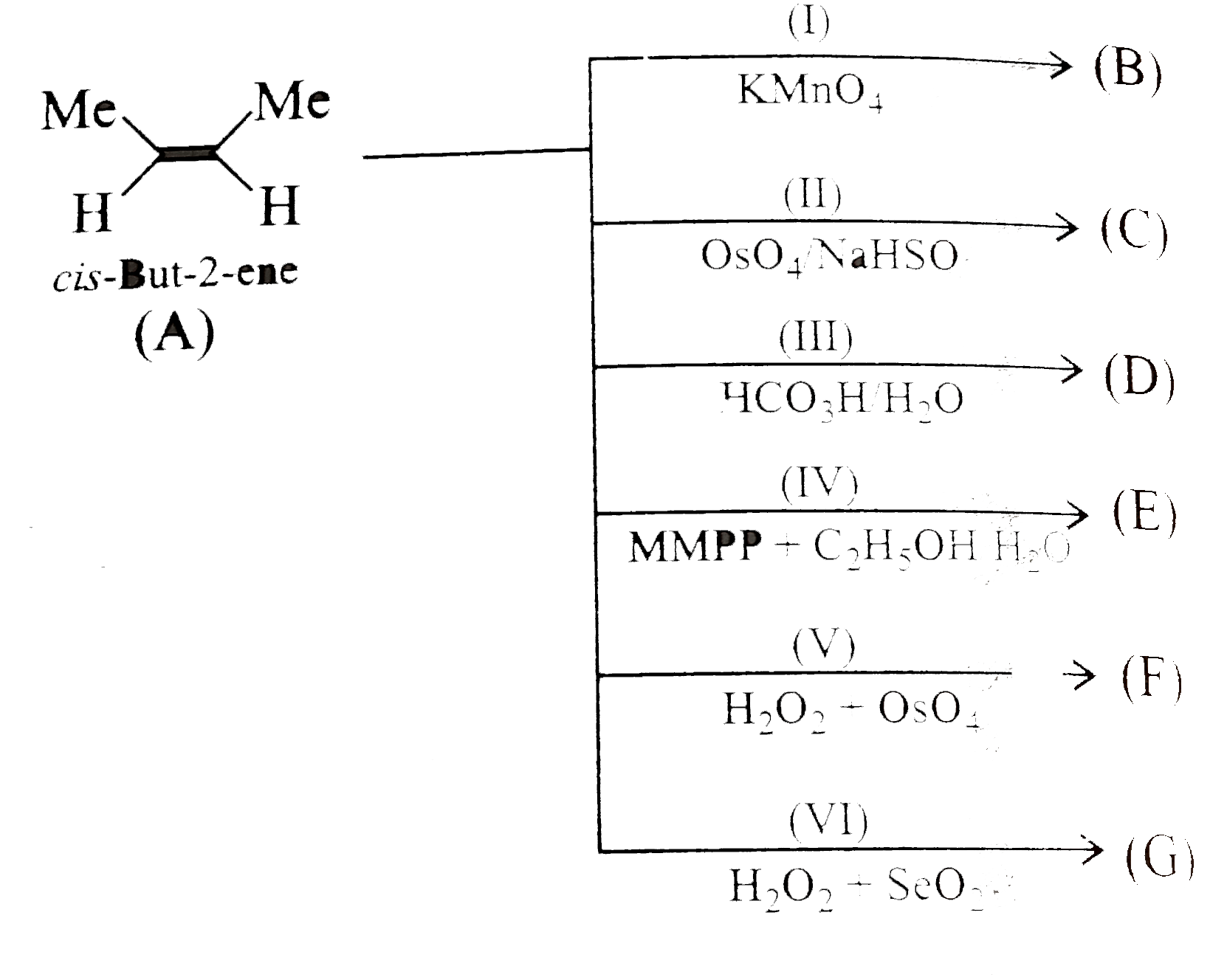
Syn-addition takes place in :
View SolutionConsider the following reactions :
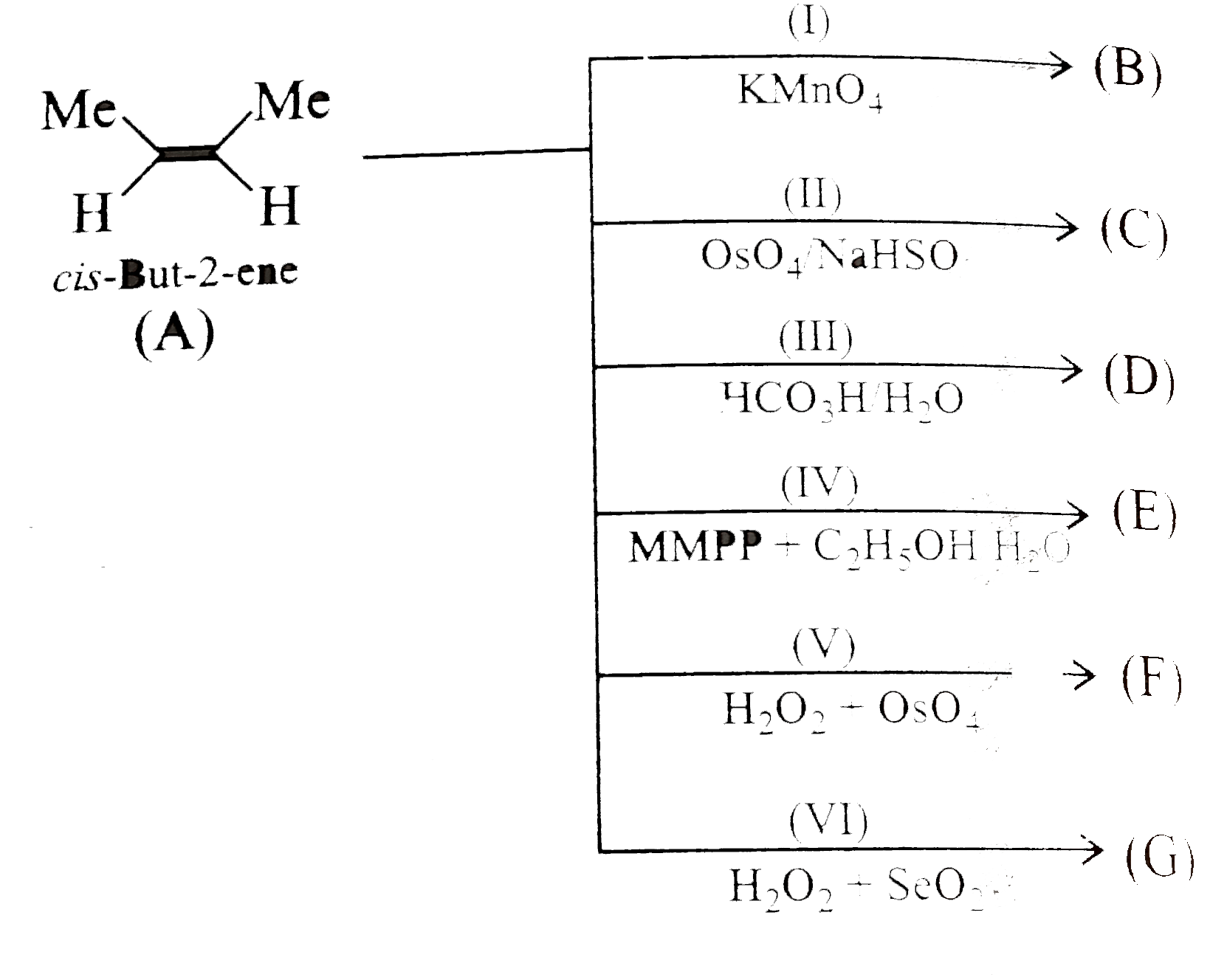
Syn-addition takes place in :
View SolutionConsider the following reactions :
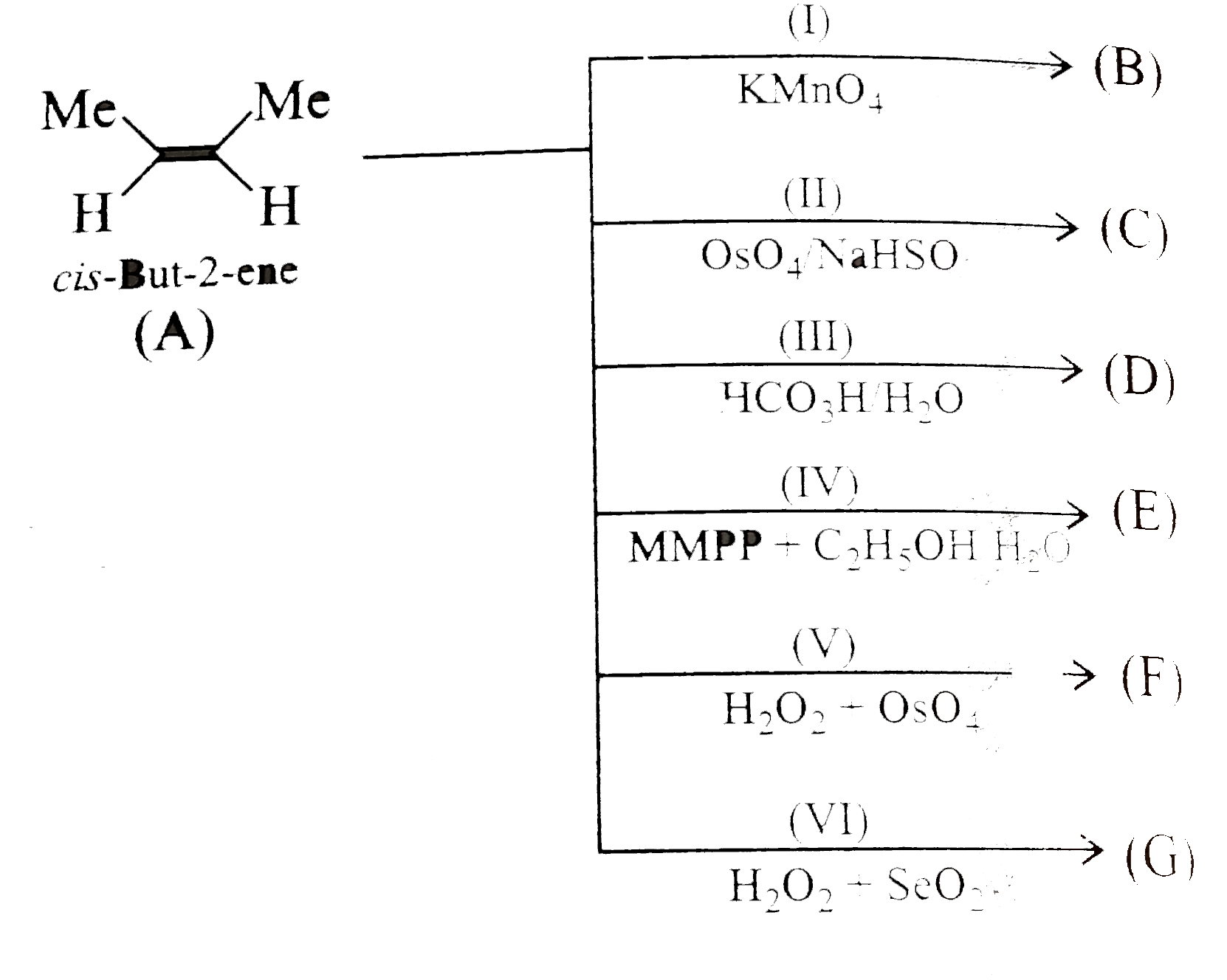
Anti-addition takes place in :
View Solution
