[A] : The energy derived from enzyme-sub-strate interaction , is called energy of formation .
[R] : Free energy is the major source of energy used by enzymes to lower the activation energies of reactions .
Activation energy
Binding energy
Constant energy
Varible energy.
The correct Answer is:B
Step by step video solution for [A] : The energy derived from enzyme-sub-strate interaction , is called energy of formation . [R] : Free energy is the major source of energy used by enzymes to lower the activation energies of reactions . by Biology experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- MOLECULES OF THE CELLBook:DINESH PUBLICATION ENGLISHChapter:MOLECULES OF THE CELLExercise:CYG
Explore 43 Videos
- MOLECULES OF THE CELLBook:DINESH PUBLICATION ENGLISHChapter:MOLECULES OF THE CELLExercise:CYG
Explore 43 Videos
- MICROBES IN HUMAN WELFAREBook:DINESH PUBLICATION ENGLISHChapter:MICROBES IN HUMAN WELFAREExercise:Brain Teasers-VIII
Explore 60 Videos
- MOLLUSCABook:DINESH PUBLICATION ENGLISHChapter:MOLLUSCAExercise:CYG
Explore 10 Videos
Similar Questions
Immediate source of energy is
View SolutionImmediate source of energy is
View SolutionHuman society started use of bronze/copper
View SolutionSiphuncle or living tube occurs in
View SolutionEnzymes catalyse the biochemical reactions by ____ the activation energy.
View SolutionWhich of the following statements about enzymes are correct ?
(i) Enzymes do not alter the overall change in free energy for a reaction.
(ii) Enzymes are proteins whose three dimensional energy.
(iii) Enzymes speed up reactions by lowering activation energy.
(iv) Enzymes are highly specific for reactions.
(v) The energy input needed to start a chemical reaction is called activation energy.
View SolutionEnergy relesed from enzymes-substrate interaction is
View SolutionAssertion- Enzymes lowers the activation energy of the reaction. Reason- Higher activation energy helps the molecules to react with greater rate.
View SolutionHow the presence of an enzyme affects the activation energy of a reaction?
View SolutionAn enzyme catalyses a reaction by activation energy of the reaction .
View SolutionWhat is denoted by X and Y in the given graph?
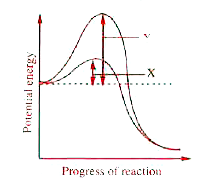
XY1) Activation energy without enzymeActivation energy with enzyme2) Activation energy with enzyme Activation energy without enzyme3) Substrate concentration with enzymeSubstrate concentration without enzyme4) Substrate concentration without enzymeSubstrate concentration with enzyme
View SolutionWhich of the following statements about enzymes are correct ?
(i) Enzymes do not alter the overall change in free energy for a reaction.
(ii) Enzymes are proteins whose three dimensional energy.
(iii) Enzymes speed up reactions by lowering activation energy.
(iv) Enzymes are highly specific for reactions.
(v) The energy input needed to start a chemical reaction is called activation energy.
View SolutionIn the presence of an enzyme, the activation energy of a reaction
View Solution[A] : The energy derived from enzyme-sub-strate interaction , is called energy of formation . [R] : Free energy is the major source of energy used by enzymes to lower the activation energies of reactions .
View SolutionDiscuss 'Enzymes lower the activation energy'.
View SolutionIn the presence of an enzyme, the activation energy of a reaction
View Solution
