Which of the following compounds have more than one type of hybrid carbon?
CH=CH−CH=CH2
HC=C−C≡CH
CH3−CH2−CH2−CH3
CH3−C≡CH3
The correct Answer is:D
CH3−C≡C−CH3 sp3 sp sp sp3
Topper's Solved these Questions
- NOMENCLATURE OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:NOMENCLATURE OF ORGANIC COMPOUNDSExercise:CONSOLIDATED EXERCISE
Explore 8 Videos
- NOMENCLATURE OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:NOMENCLATURE OF ORGANIC COMPOUNDSExercise:CHALLENGING EXERCISE
Explore 15 Videos
- INTRODUCTION TO ORGANIC CHEMISTRYBook:CENGAGE CHEMISTRYChapter:INTRODUCTION TO ORGANIC CHEMISTRYExercise:OLYMPIAD AND NTSE LEVEL EXERCISES
Explore 13 Videos
- ORGANIC COMPOUNDS CONTAINING OXYGEN ATOM Book:CENGAGE CHEMISTRYChapter:ORGANIC COMPOUNDS CONTAINING OXYGEN ATOM Exercise:OLYMPIAD AND NTSE LEVEL EXERCISES
Explore 10 Videos
Similar Questions
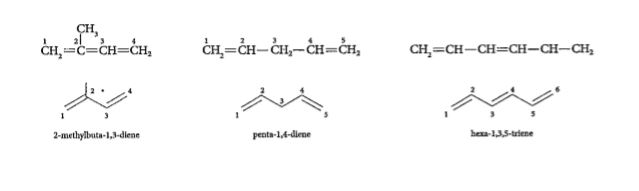
Comprehension Bond line notation of organic molecules It is a simple and convenient method of representing structures. In this notation, bonds are represented by lines and carbon atoms by line ends and intersections. It is assumed that the required number of H atoms are present wherever necessary to satisfy the tetracovalency of carbon. For example, 2-methylbuta-1,3-diene, penta-1,4-diene and hexa-1,3,5-triene may be represented as follows:
Rewrite the following structural formulae in bond line notation :
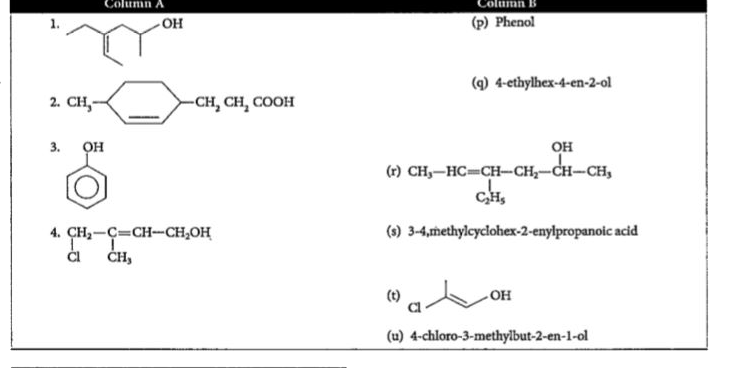
View SolutionMatch the following
View SolutionWhich of the following statements is not applicable to carbon compounds?
View SolutionPrimary and secondary alcohols on action of reduced copper give
View SolutionWhich of the following compounds contains more than one kind of hybridized carbons?
View SolutionWhich of the following compounds has (have) sp-hybridized carbon atom?
View SolutionIn which of the following pairs of compounds, will second structure have more contribution to resonance hybrid than first ?
View Solutionकिन योगिकों में कार्बन के प्रसंकरण एक प्रकार के हैं?
View SolutionAmong the following compounds which have more than one type of hybridisation for carbon atoms.
View SolutionWhy are the type of hybridization of each carbon in the following compound? hequivC-CH=CH_2
View SolutionWhich of the following compounds does not have only one type of hybrid carbon atoms ?
View SolutionWhich of the following compound does not have only one type of hybrid carbon atoms?
View SolutionNaming of Compounds having more than One type of Functional Groups
View SolutionAmong the following compounds which does not have more than one type of hybridisation for carbon atoms?
View SolutionThe compound having only one type of hybrid obitals in all carbon atoms is
View SolutionIn which of the following the hybridisation of carbon is more than one?
View Solution

