How many geometrical isomers are possible in the following co-ordination entity?
[Co(NH3)3Cl3]Solution in Punjabi
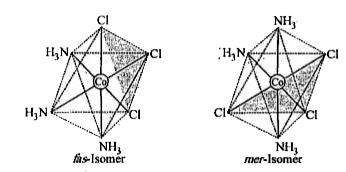
[Co(NH3)3Cl3] : Two geometrical isomers (fac and mer) are possible.
Topper's Solved these Questions
- CO-ORDINATION COMPOUNDSBook:BETTER CHOICE PUBLICATIONChapter:CO-ORDINATION COMPOUNDSExercise:QUESTION FROM PREVIOUS BOARD EXAMINATION
Explore 59 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:BETTER CHOICE PUBLICATIONChapter:CHEMISTRY IN EVERYDAY LIFEExercise:QUESTIONS
Explore 76 Videos
- ELECTROCHEMISTRYBook:BETTER CHOICE PUBLICATIONChapter:ELECTROCHEMISTRYExercise:Numerical Problems
Explore 69 Videos
Similar Questions
Write the IUPAC name of corordination compound [Co(NH3)3ONO]Cl2.
View SolutionWhat is a medicine ?
View SolutionDerive Nernst equation for the following cell:
NiNi2+(0⋅2M)∣∣∣Cu2+(0⋅2M)∣∣Cu.
View SolutionHow many geometrical isomers are possible in the following corrdination entities? (i). [Cr(C_2O_4)_3]^(3-) (ii). [Co(NH_3)_3Cl_3]
View SolutionHow many geometrical isomers are possible [Co(acac)2BrCl]Θ are:
View SolutionHow many geometrical isomers are possible in the following co-ordination entities?
(i) [Cr(C2O4)3]3−
(ii) [Co(NH3)3Cl3]
View SolutionHow many geometrical isomers are possible [Pt(py)(NH_3) (Br)(Cl)]?
View SolutionHow many isomers are possible for [Co(NH_3)_4Cl_2]Cl ? Draw their structures.
View SolutionThe number of geometric isomers of the complex Cr(NH_3)_3Cl_3 are
View SolutionHow many geometrical isomers are possible [Co(acac)_(2)BrCl]^(Theta) are:
View SolutionHow many geometrical isomers are possible in the following corrdination entities? (i). [Cr(C_2O_4)_3]^(3-) (ii). [Co(NH_3)_3Cl_3]
View SolutionThe coordination number of cobalt in [Co(NH3)3Cl3] is
View SolutionThe coordination number of cobalt in [Co(NH3)3Cl3] is
View SolutionHow many geometrical isomers are possible in the following co-ordination entities?
[Cr(C2O4)3]3−
View SolutionHow many geometrical isomers are possible in the following co-ordination entities?
[Cr(C2O4)3]3−
View SolutionHow many isomers are possible for [Co(NH_3)_4CI_2 ? Draw their struc-tures.
View Solution
