From the following flowsheet for the extraction of pure metal, answer the given questions.
(vi) following reaction is not involved intermite process :
3Mn3O4+8Al→9Mn+4Al2O3
Cr2O3+2Al→Al2O3+2Cr
2Fe+Al2O3→2Al+Fe2O3
B2O3+2Al→2B+Al2O3
The correct Answer is:C
In themite welding
Fe2O3(s)+2AI(s)Δ−−→MgFe(l)+AI2O3(l)
Topper's Solved these Questions
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE-03
Explore 36 Videos
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE -04
Explore 54 Videos
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE-01
Explore 41 Videos
- ISOMERISM Book:ALLENChapter:ISOMERISM Exercise:MCQ
Explore 20 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:J-ADVANCE
Explore 2 Videos
Similar Questions
State True/False
Metals can be recovered from their ores by chemical methods.
View SolutionIn the extraction of chlorine by electrolysis of brine ___________ .
View SolutionName :
The most abundant elements in the earth's crust .
View SolutionCH3CH2CH(CHO)CH2CH3and
CH3CH(CHO)CH2CH2CH3 are :-
View SolutionAmmonia prepared by treating ammonium sulphate with calcium hydroxide is completely used NiCl2.6H2O to form a stable coordination compound. Assume that both the reactions are 100% complete. If 1584 g of ammonium sulphate and 952 g of NiCl2.6H2O are used in the preparation, the combined weight (in grams) of gypsum and the nickel - ammonia coordination compound thus produced is _____.
(Atomic weights in g mol−1 : H=1,N=14,O=16 , S=32, Cl=35.5 , Ca=40 , Ni=59)
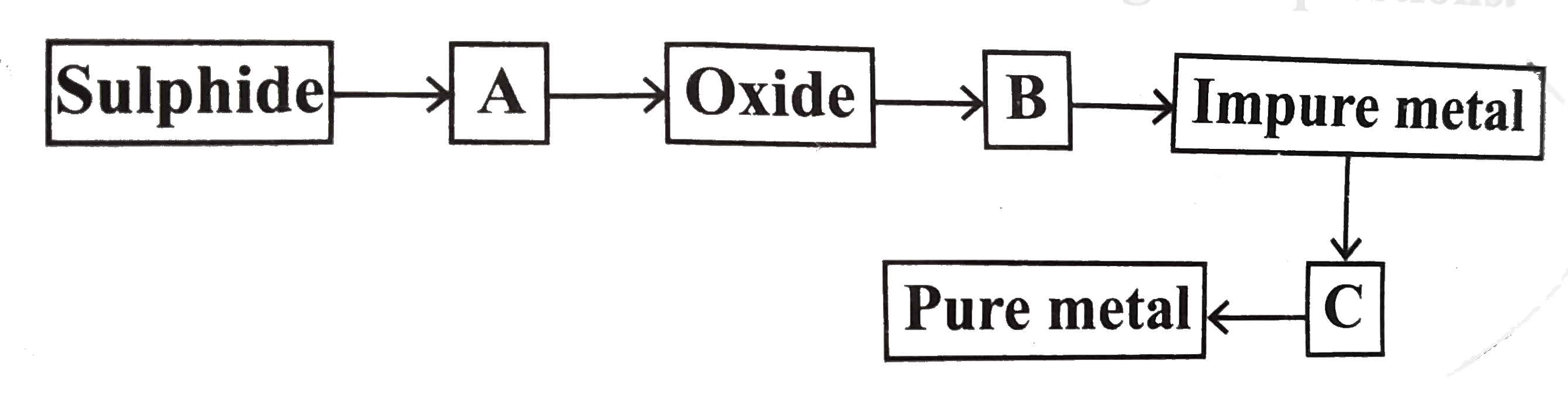
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions. (i) Step A is :
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
(ii) Step B (reduction) can be carried out ny using :
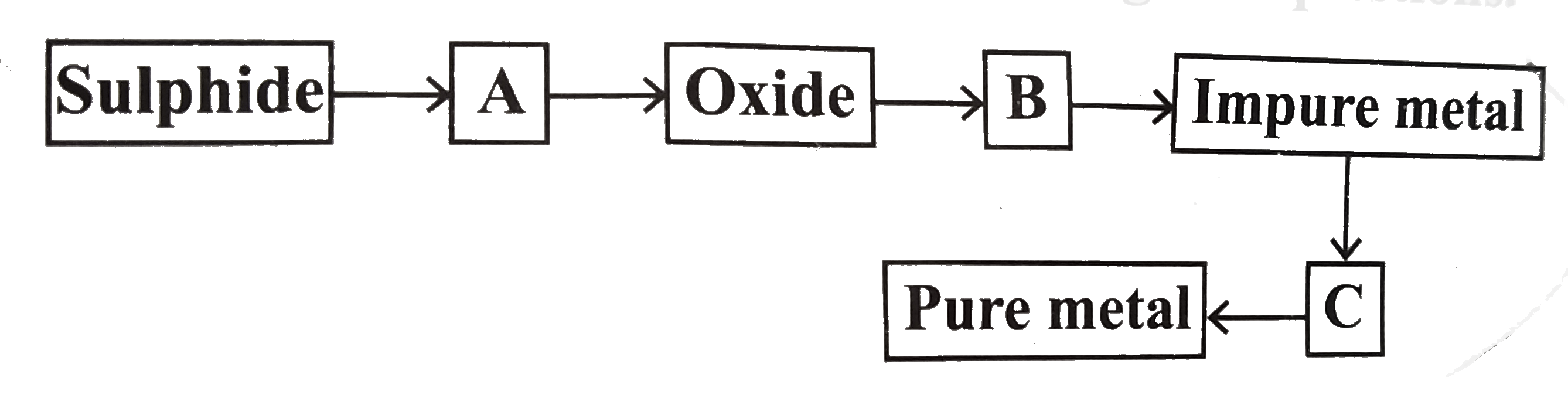
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
(iii) Impure Cu metal is also obtained :
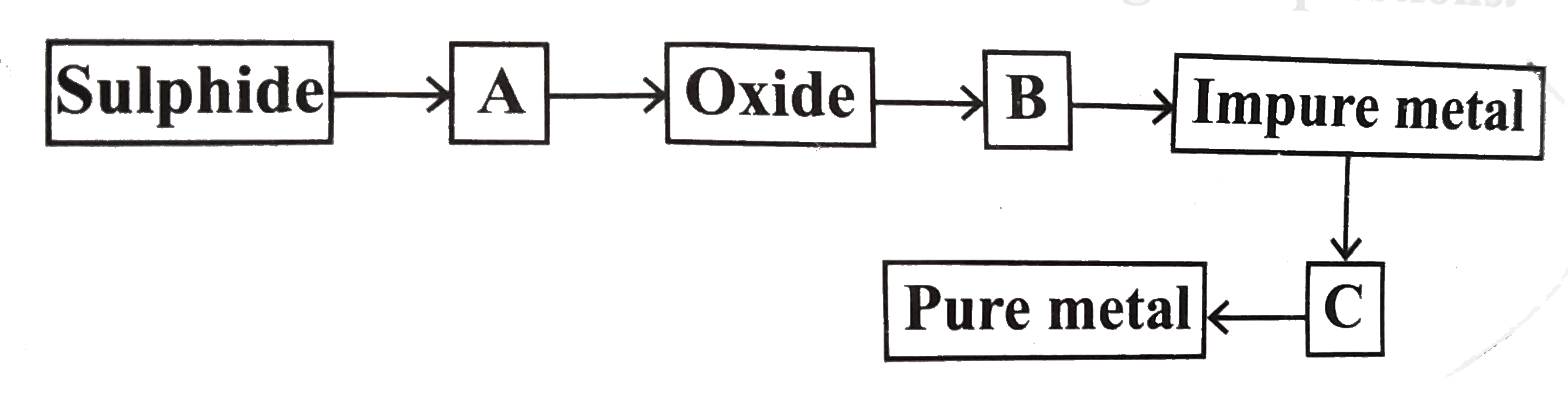
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
(iv) Some of the following metals are obtained by electrolysis of their fused salts : Al,Na,Cu,Ag,Mg,Ca. These metals are :
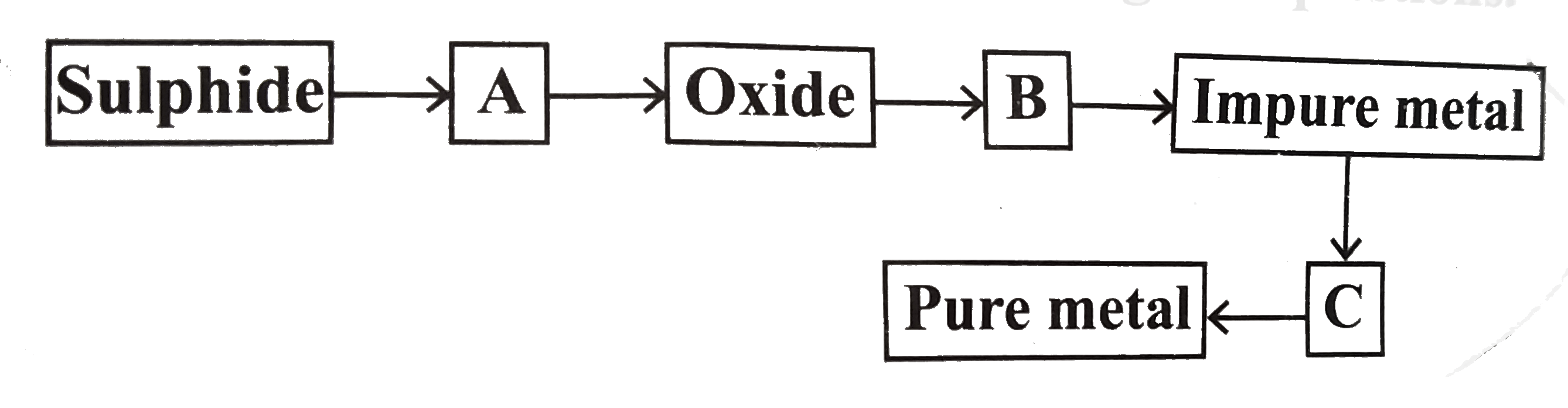
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
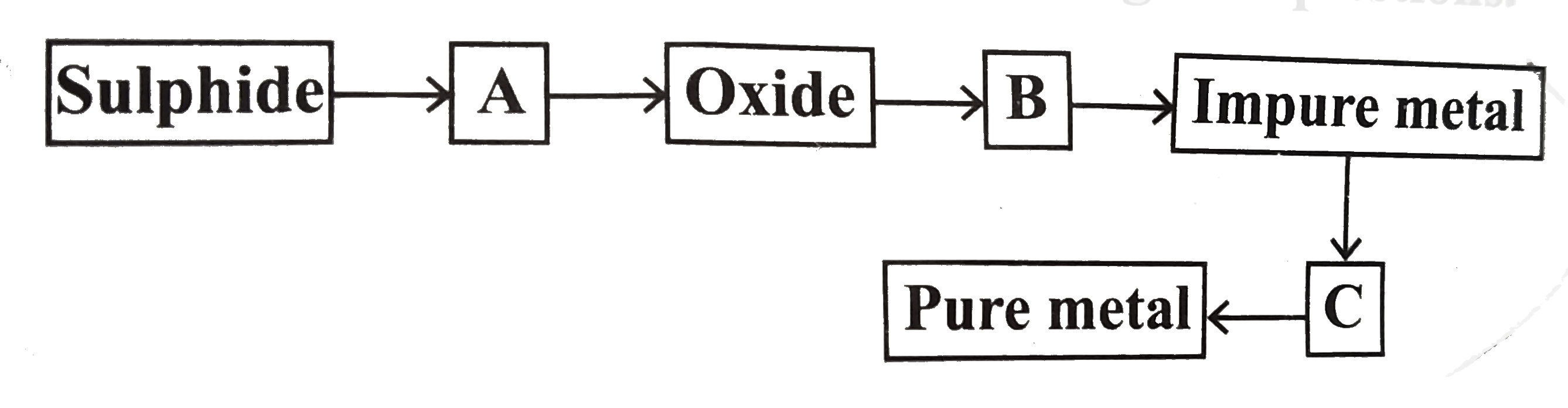
(v) Reduction of oxides to elements with carbon generally takes place at high temperature, hence Al is used in aluminothermite process. It is because :
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions. (vi) following reaction is not involved intermite process :
View SolutionWhat are the various steps involved in extraction of pure metals from their ores ?
View SolutionWhat are the various steps involved in extraction of pure metals from their ores ?
View SolutionWhat are the various steps involved in extraction of pure metals from their ores ?
View SolutionLook at the following diagram and answer the questions given below :
Write the overall reaction taking place in the extraction of metal.
View SolutionIn the stages involved in the extraction of metals in general - give reasons for following Dressing of the ore is an essential process in the extraction of metal from its ore.
View Solution
