Extraction of aluminium can be understood by :
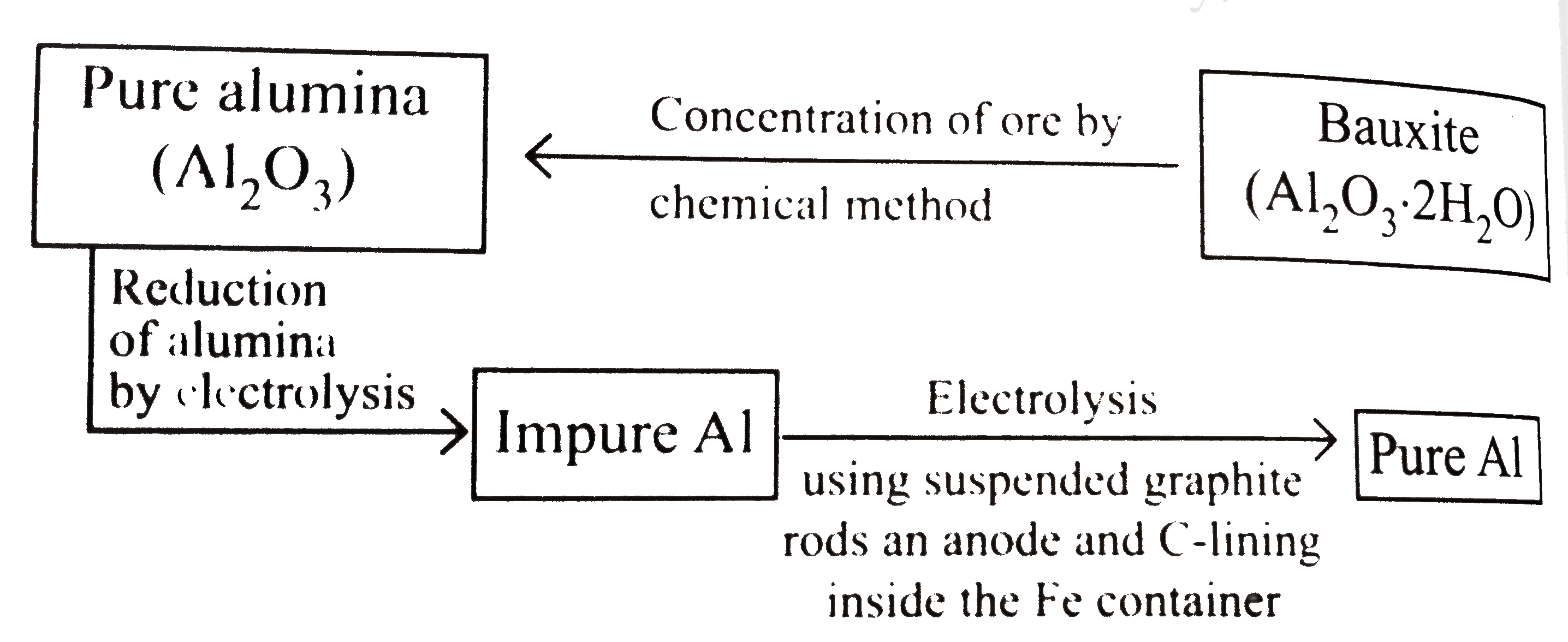
Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The molten electrolytes contain Na⊕,Al3+ and Ca2+ but only Al gets deposited at cathode because,
Standard reduction potential of Al is more than those of Na&Ca
Standard oxidation potential of Al is more than those of Na&Ca
Graphite reacts only with Al3+ and not with Na+&Ca2+
Discharge potential of Al3+ is higher than Na+&Ca2+
The correct Answer is:A
Step by step video solution for Extraction of aluminium can be understood by : Electrolytric reduction of Al_(2) O_(3) : Electrolysis : Al_(2) O_(3) + Cryolite + CaF_(2) Cathode : Carbon inside the Fe container Anode : Graphite rods. The molten electrolytes contain Na^(oplus),Al^(3+) and Ca^(2+) but only Al gets deposited at cathode because, by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- METALLURGYBOOK - ALLENCHAPTER - METALLURGYEXERCISE - EXERCISE -04
54 Videos
- METALLURGYBOOK - ALLENCHAPTER - METALLURGYEXERCISE - EXERCISE-05 [A]
11 Videos
- METALLURGYBOOK - ALLENCHAPTER - METALLURGYEXERCISE - EXERCISE-02
45 Videos
- ISOMERISM BOOK - ALLENCHAPTER - ISOMERISM EXERCISE - MCQ
20 Videos
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - J-ADVANCE
2 Videos
Similar Questions
In the extraction of chlorine by electrolysis of brine ___________ .
View SolutionAluminium is extracted from bauxite by the electrolysis of aluminium oxide dissolved in cryolite. Now answer the following question:
Write the formula of cryolite.
View SolutionStatement-1:In cyanide process for the extraction of gold and silver from their native ores, the cyanide solution acts as a reducing agent
to reduce the gold and silver compounds present in the ores, into
the metallic states.
Statement-2:In the extraction of gold and silver the cyanide solution acts as complexing reagent in the presence of air and form their respective soluble complexes.
View SolutionCH3CH2CH(CHO)CH2CH3and
CH3CH(CHO)CH2CH2CH3 are :-
View SolutionAmmonia prepared by treating ammonium sulphate with calcium hydroxide is completely used NiCl2.6H2O to form a stable coordination compound. Assume that both the reactions are 100% complete. If 1584 g of ammonium sulphate and 952 g of NiCl2.6H2O are used in the preparation, the combined weight (in grams) of gypsum and the nickel - ammonia coordination compound thus produced is _____.
(Atomic weights in g mol−1 : H=1,N=14,O=16 , S=32, Cl=35.5 , Ca=40 , Ni=59)
View SolutionExtraction of aluminium can be understood by :
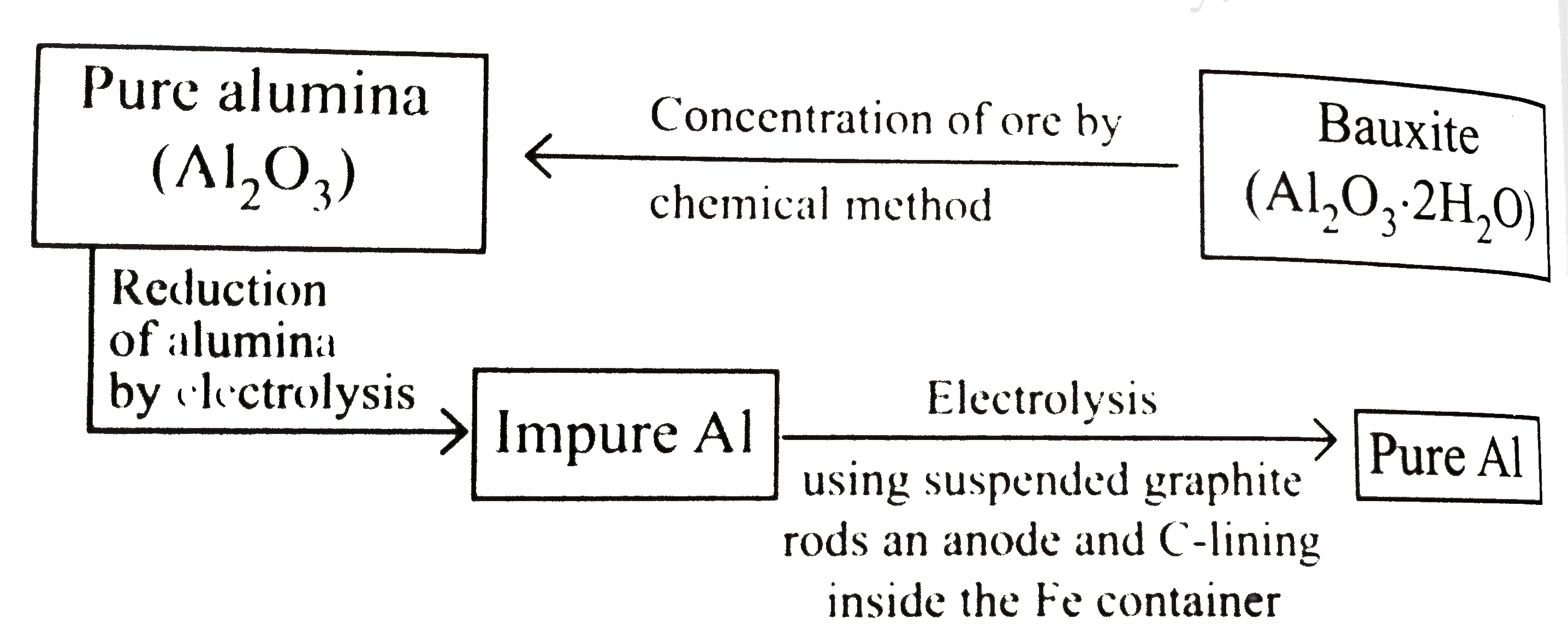
Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :
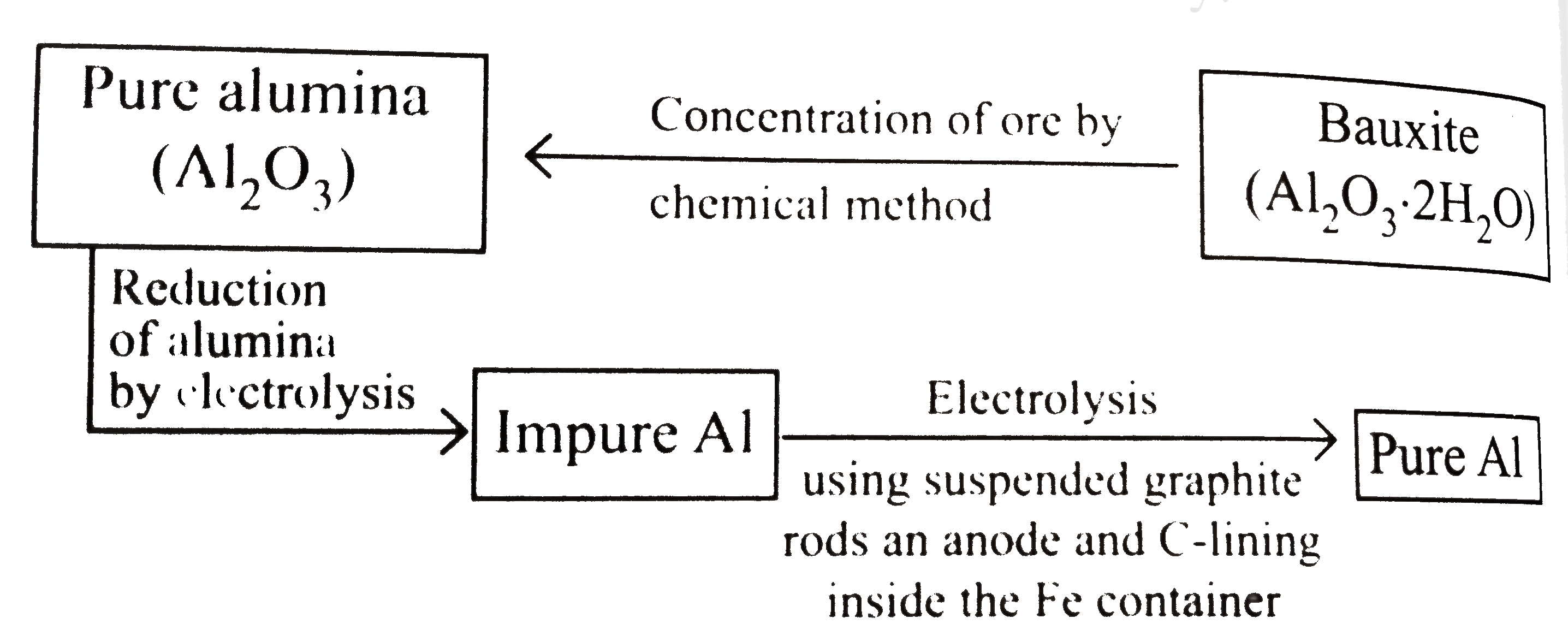
Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
Coke power is spread over the molten electroltyte to.
View SolutionExtraction of aluminium can be understood by : Electrolytric reduction of Al_(2) O_(3) : Electrolysis : Al_(2) O_(3) + Cryolite + CaF_(2) Cathode : Carbon inside the Fe container Anode : Graphite rods. The function of fluorspar (CaF_(2)) is.
View SolutionExtraction of aluminium can be understood by : Electrolytric reduction of Al_(2) O_(3) : Electrolysis : Al_(2) O_(3) + Cryolite + CaF_(2) Cathode : Carbon inside the Fe container Anode : Graphite rods. The motion electrolytes contain Na^(oplus),Al^(3+) and Ca^(2+) but only Al gets deposited at cathode because,
View SolutionElectrolytic reducation of Al_(2)O_(3) Electrolyte (Al_(2)O_(3)+cryolite) Cathode : fraphite inside Fe -Container Pick out the statement which is wrong if anode is made of nikel instesd of graphoite?
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
Coke power is spread over the molten electroltyte to.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of Aluminiunt can be understood by : Electrolytic reduction of Al_(2)O_(3) : {:("Electrolyte",:(Al_(2)O_(3)+"Cryolite")),("Cathode",:"Graphite inside the Fe container"),("Anode",:"Graphite rods"):} The purpose of adding cryolite is
View SolutionExtraction of Aluminiunt can be understood by : Electrolytic reduction of Al_(2)O_(3) : {:("Electrolyte",:(Al_(2)O_(3)+"Cryolite")),("Cathode",:"Graphite inside the Fe container"),("Anode",:"Graphite rods"):} Coke powder is spreaded over the molten electrolyte to
View Solution

