Give reasons for the following:
a. Alkali metals do not occur free in nature.
b. Alkali metal salts impart characteristic colour to the flame.
c. Caesium is used in photoelectric cell.
d. Alkali metals are good reducing agents in aqueous medium.
Oxidising flame
Reducing flame
Both a & b
None of these
The correct Answer is:A
Step by step video solution for Give reasons for the following: a. Alkali metals do not occur free in nature. b. Alkali metal salts impart characteristic colour to the flame. c. Caesium is used in photoelectric cell. d. Alkali metals are good reducing agents in aqueous medium. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- Chemical EquilibriumBOOK - ALLENCHAPTER - Chemical EquilibriumEXERCISE - All Questions
30 Videos
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE -05 [B]
38 Videos
Similar Questions
The correct IUPAC name of the following compound is :
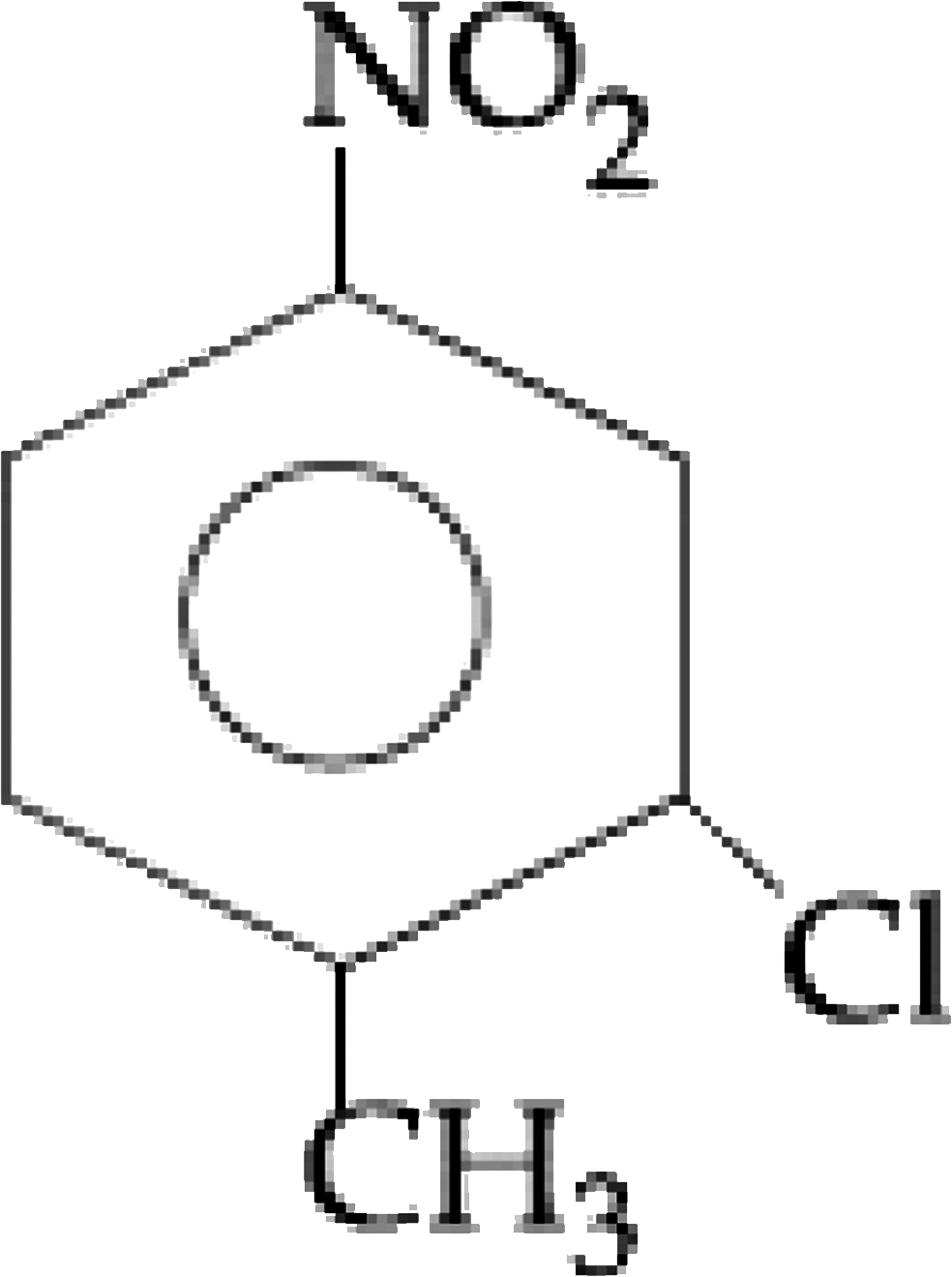
View SolutionThe correct IUPAC name of the following compound is :

View Solution48 g of SO3 , 12.8 g of SO2 and 9.6 g of O2 are present in one litre . The respective active masses will be
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View SolutionGive reasons for the following:
a. Alkli metals do not occur free in nature.
b. Alkali metal salts impart characteristic colour to the flame.
c. Caesium is used in photoelectric cell.
d. Alkali metals are good reducing agents in aqueous medium.
View SolutionThe alkali metals & their salts impart characteristic colour to an:
View SolutionAlkali metals impart colour to Bunsen flame due to
View SolutionAssign reasons for the following (i) alkali metals impart characteristic colour to flame (ii) Alkali metals are strong reducing agents. (iii) An aqueous solution of Na_(2)CO_(3) is basic in nature.
View SolutionWhy does alkali metals impart characteristic colour to the flame?
View SolutionWhy do some of the alkali metal salts impart colour to the flame?
View SolutionAlkali metals and their salts impart characteristic colours to an oxidizing flame. Explain the reason.
View SolutionWhy do alkali metals impart characteristic colour to the flame of bunsen burner?
View Solutionwhy do alkali metals give characteristic flame colouration?
View SolutionWhy the alkali metals and their salts impart characteristic colour to an oxidizing flame ?
View SolutionHow do alkali metal salts give characteristic flame colours?
View SolutionThe alkali metals and their salts impart characteristic colour to an oxidising flame.What is the reason for this?
View Solution

