Which of the following will have three stereoisomeric forms?
(i) [Cr(NO3)3(NH3)3]
(ii) K3[Co(C2O4)3]
(iii) K3[CoCl2(C2O4)2]
(iv) [CoBrCl(en)2]
iii, iv
I, ii and iv
Only iv
All
The correct Answer is:A
Step by step video solution for Which of the following will have three stereoisomeric forms? (i) [Cr(NO_(3))_(3)(NH_(3))_(3)] (ii) K_(3)[Co(C_(2)O_(4))_(3)] (iii) K_(3)[CoCl_(2)(C_(2)O_(4))_(2)] (iv) [CoBrCl(en)_(2)] by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- Chemical EquilibriumBOOK - ALLENCHAPTER - Chemical EquilibriumEXERCISE - All Questions
30 Videos
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE -05 [B]
38 Videos
Similar Questions
The correct IUPAC name of the following compound is :
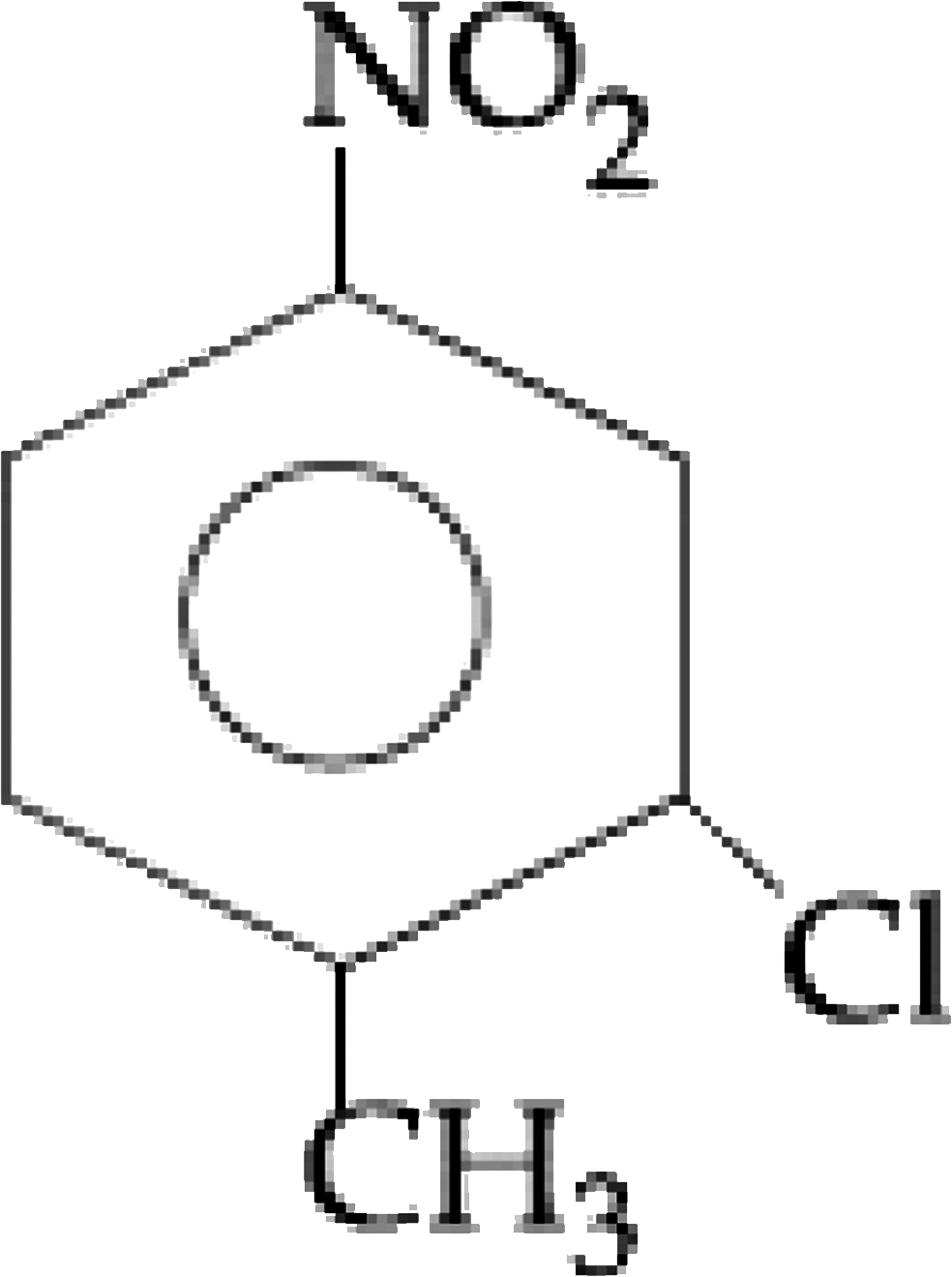
View SolutionThe correct IUPAC name of the following compound is :

View Solution48 g of SO3 , 12.8 g of SO2 and 9.6 g of O2 are present in one litre . The respective active masses will be
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View SolutionWrite the IUPAC names of the following coordination compounds:
(a)[Pt(NH3)2Cl(NO2)]
(b) K3[Cr(C2O4)3]
(c) [CoCl2(en)2]Cl
(d) [Co(NH3)]Cl
(e) Hg[Co(SCN)4]
View SolutionWhich of the following will have three stereoisomeric forms :-
(i) [Cr(NO3)3(NH3)3] (ii) K3[Co(C2O4)3]
(iii) K3[CoCl2(C2O4_(2)] (iv) [CoBrCl(en)2]
View Solutionनिम्नलिखित में केन्द्रीय धातु परमाणु की ऑक्सीकरण संख्या ज्ञात कीजिए |
(a) Fe2(CO)9
(b) [Fe(EDTA)]−1
(c ) [Co(NO2)2(py)2(NH3)2]NO3
(d) [Co(NH3)6]Cl3
(e ) K3[Cr(C2O4)3]
(f) K3[CoF4]
(g) K2[HgI4]
(h) K3[Co(C2O4)2Cl2]
View Solutionनिम्नलिखित के IUPAC नाम लिखिए -
(i) [Cu(NH3)4]SO4
(ii) [Co(NH3)2Cl2(en)]+
(iii) [CoCl2(en)2]
(iv) K3[Al(C2O4)3]
(v) [Co(NH3)6][CdCl5].
View Solutionनिम्न प्रकाशित समावयवो के संरचनाएं बनाइए -
(i) [Cr(C2O4)3]3−, (ii) [PtCl2(en)2]2+, (iii) [Cr(NH3)2Cl2(en)]+.
View Solutionनिम्न के प्रकाशिक़ समावयवों की संरचनाएं बनाइये।
i) [Cr(C2O4)3]3−,
ii) [PtCl2(en)2]2+
iii) [Cr(NH3)2Cl2(en)]+
View SolutionWhich of the following will have two stereoisomeric forms? (I) [Cr(NO_(3))_(3)(NH_(3))_(3)] (II) K_(3)[Fe(C_(2)O_(4))_(3)] (III) [CoCl_(2)(en)_(2)]^(+) (IV) [CoBrCl(o x)_(2)]^(3-)
View SolutionWhich of the following will have three stereoisomeric forms?
(i) [Cr(NO3)3(NH3)3]
(ii) K3[Co(C2O4)3]
(iii) K3[CoCl2(C2O4)2]
(iv) [CoBrCl(en)2]
View SolutionWrite the IUPAC names of the following coordination compounds : (a) [Pt(NH_(3))_(2)Cl(NO)_(2)] (b) K_(3)[Cr(C_(2)O_(4))_(3)] (c) [CoCl_(2)(en)_(2)]Cl (d) [Co(NH_(3))_(5)(CO_(3))]Cl (e) Hg[Co(SCN)_(4)]**
View SolutionDraw the structures of optical isomers of (i) [Cr(C_(2)O_(4))_(3)]^(3-) (ii) [PtCl_(2)(en)_(2)]^(2+) (iii) [Cr(NH_(3))Cl_(2)(en)]^(+)
View SolutionWrite the IUPAC names of the following coordination compounds (a) [Pt(NH_(3))_(2)CH(NO_(2)) ( (b) K_(3)Cr(C_(2)O_(3))] C) [CoCl_(2)(en)_( 2)]CI (d). [CO(NH_(3))_(5)(CO_(3)]CI (e) Hg[Co(SCN)_(4)]
View SolutionWhich of the following will have two stereoisomeric forms?
(I) [Cr(NO3)3(NH3)3]
(II) K3[Fe(C2O4)3]
(III) [CoCl2(en)2]+
(IV) [CoBrCl(ox)2]3−
View Solution

