Which of the following change is possible in aqueous medium -
2Cu+→Cu2++Cu0
2Cu0−−−→−3e−Cu++Cu+2
2Cu+2−−−→+3e−Cu0+Cu+
Cu+2+e−→Cu+
The correct Answer is:A
To determine which of the given changes is possible in an aqueous medium, we need to analyze the standard reduction potentials (E°) for the reactions involved. Here’s a step-by-step breakdown of the solution:
Step 1: Identify the Reactions and Their Standard Reduction Potentials
We have the following reactions with their respective standard reduction potentials:
1. Cu2++2e−→Cu (E° = +0.34 V)
2. Cu++e−→Cu (E° = +0.52 V)
3. 2H2O→O2+4H++4e− (E° = -1.23 V)
4. 2H++2e−→H2 (E° = -0.828 V)
Step 2: Calculate the Standard Cell Potential for Each Reaction
We will calculate the cell potential (E) for each proposed reaction.
Reaction A: 2Cu+→Cu2++Cu
- For Cu+→Cu2++e− (reverse of reduction): E° = -0.52 V
- For Cu2++2e−→Cu: E° = +0.34 V
- Total E = -0.52 V + 0.34 V = -0.18 V (not feasible)
Reaction B: Cu→Cu++Cu2+
- For Cu→Cu++e−: E° = -0.52 V
- For Cu→Cu2++2e−: E° = -0.34 V
- Total E = -0.52 V + -0.34 V = -0.86 V (not feasible)
Reaction C: Cu2++2e−→Cu+
- For Cu2++2e−→Cu: E° = +0.34 V
- For Cu++e−→Cu: E° = +0.52 V
- Total E = +0.34 V - 0.52 V = -0.18 V (not feasible)
Reaction D: Cu2++e−→Cu+
- For Cu2++2e−→Cu: E° = +0.34 V
- For Cu++e−→Cu: E° = +0.52 V
- Total E = +0.34 V - 0.52 V = -0.18 V (not feasible)
Step 3: Determine Feasibility
- A reaction is feasible if the cell potential (E) is greater than 0.
- From our calculations, all reactions resulted in negative potentials, indicating that none of the reactions are feasible in an aqueous medium.
Conclusion
None of the proposed changes are possible in an aqueous medium based on the calculated standard reduction potentials.
We have the following reactions with their respective standard reduction potentials:
1. Cu2++2e−→Cu (E° = +0.34 V)
2. Cu++e−→Cu (E° = +0.52 V)
3. 2H2O→O2+4H++4e− (E° = -1.23 V)
4. 2H++2e−→H2 (E° = -0.828 V)
We will calculate the cell potential (E) for each proposed reaction.
- For Cu+→Cu2++e− (reverse of reduction): E° = -0.52 V
- For Cu2++2e−→Cu: E° = +0.34 V
- Total E = -0.52 V + 0.34 V = -0.18 V (not feasible)
- For Cu→Cu++e−: E° = -0.52 V
- For Cu→Cu2++2e−: E° = -0.34 V
- Total E = -0.52 V + -0.34 V = -0.86 V (not feasible)
- For Cu2++2e−→Cu: E° = +0.34 V
- For Cu++e−→Cu: E° = +0.52 V
- Total E = +0.34 V - 0.52 V = -0.18 V (not feasible)
- For Cu2++2e−→Cu: E° = +0.34 V
- For Cu++e−→Cu: E° = +0.52 V
- Total E = +0.34 V - 0.52 V = -0.18 V (not feasible)
- A reaction is feasible if the cell potential (E) is greater than 0.
- From our calculations, all reactions resulted in negative potentials, indicating that none of the reactions are feasible in an aqueous medium.
None of the proposed changes are possible in an aqueous medium based on the calculated standard reduction potentials.
Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
472 Videos
- Chemical EquilibriumBOOK - ALLENCHAPTER - Chemical EquilibriumEXERCISE - All Questions
30 Videos
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE -05 [B]
38 Videos
Similar Questions
The correct IUPAC name of the following compound is :
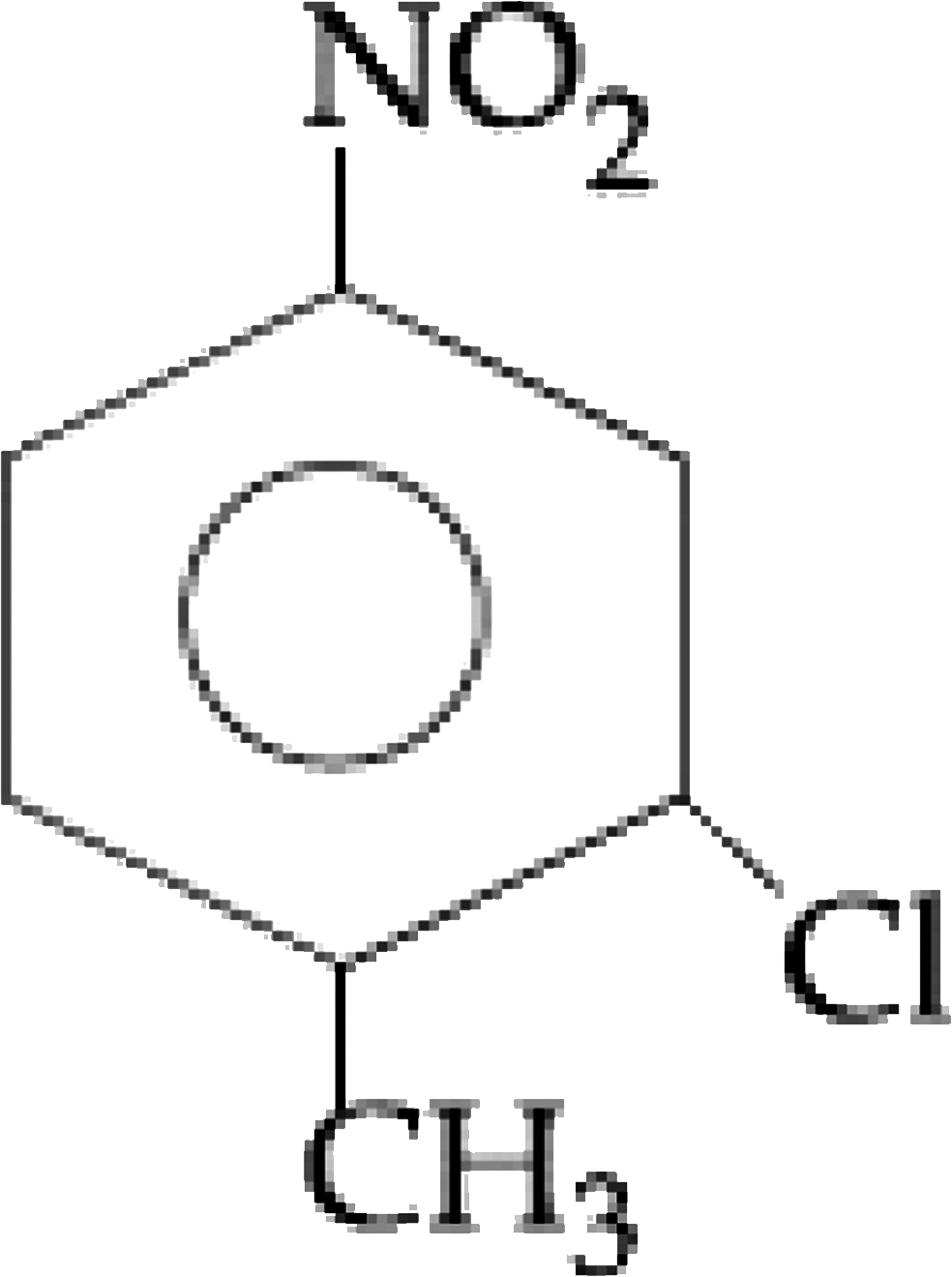
View SolutionThe correct IUPAC name of the following compound is :

View Solution48 g of SO3 , 12.8 g of SO2 and 9.6 g of O2 are present in one litre . The respective active masses will be
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View SolutionWhich of the following is the strongest reducing agent in aqueous medium?
View SolutionWhich of the following statements is true in aqueous medium?
View SolutionWhich of the following order of basicity is correct in aqueous medium?
View SolutionWhich one of the following forms a colourless solution in aqueous medium ?
(Atomic number : Sc=21,Ti=22,V=23, and Cr=24)
View SolutionWhich of the following combination of reagent(s) produce observable change in aqueous medium?
View SolutionWhich of the following is more basic in aqueous medium is
View SolutionWhich of the following is more baic in aqueous medium ?
View SolutionWhich of the following is least basic in aqueous medium ?
View SolutionWhich of the following is more baisc in aqueous medium ?
View SolutionWhich of the following forms a colourless solution in aqueous medium?
View Solutionनिम्नलिखित में जलीय माध्यम में कौन प्रबलतम अपचायक पदार्थ है ?
View Solutionनिम्नलिखित में से कौन जलीय माध्यम में सर्वाधिक क्षारीय है?
View Solution

