Which of the following compound will show optical isomerism
all of these
The correct Answer is:B
To determine which of the given compounds will show optical isomerism, we need to identify if they contain chiral centers. A chiral center is typically a carbon atom that is bonded to four different groups.
1. Identify the Compounds: We need to analyze each compound provided in the options to check for chiral centers.
2. Analyze Compound 1:
- Structure: CH3-CH(CH3)-CH2-CH(CH3)-CH3
- Identify the carbon atoms: Look for a carbon atom that has four different substituents.
- In this case, there are two carbon atoms that are chiral because they are bonded to different groups (two CH3 groups and two hydrogen atoms).
- Conclusion: Compound 1 shows optical isomerism.
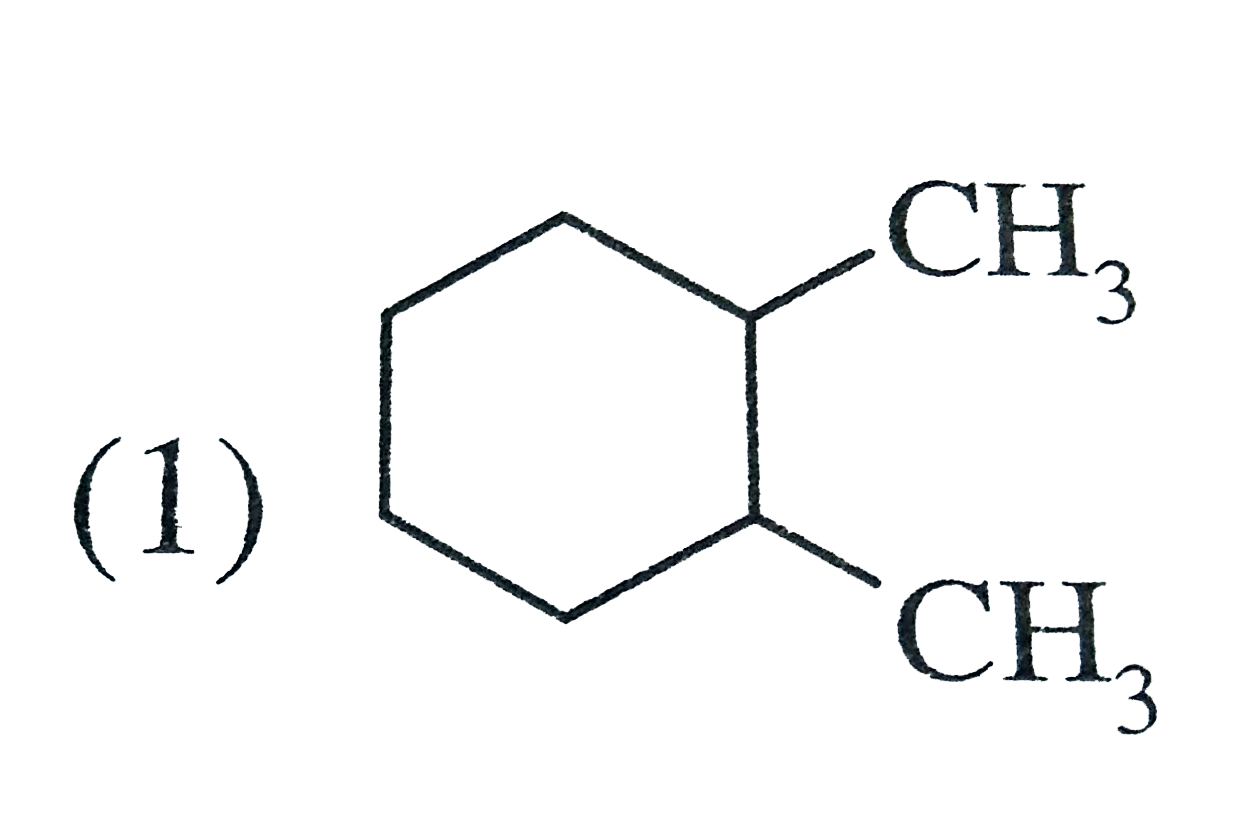
3. Analyze Compound 2:
- Structure: Cyclohexane with two CH3 groups.
- Identify the carbon atoms: Check if any carbon has four different substituents.
- Here, the carbons that have CH3 groups are not chiral because they are bonded to identical groups.
- Conclusion: Compound 2 does not show optical isomerism.
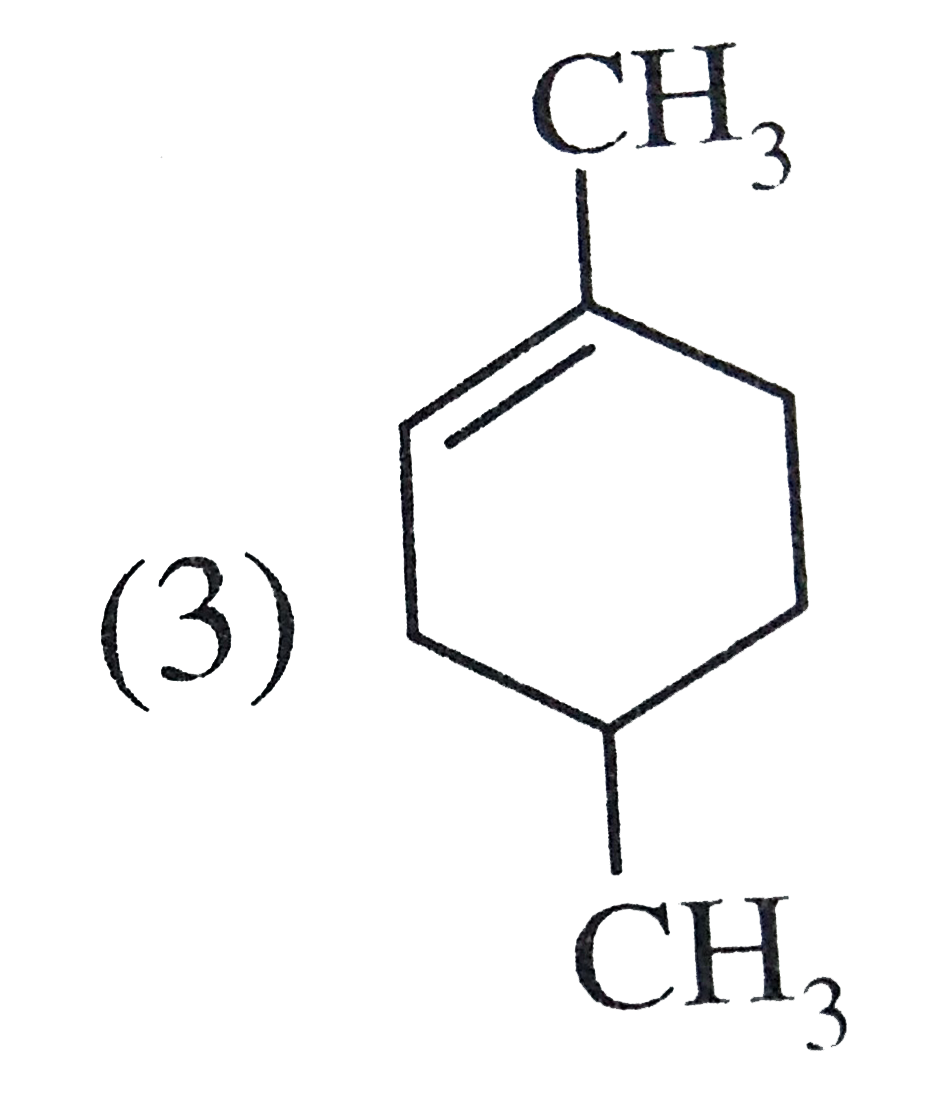
4. Analyze Compound 3:
- Structure: Cyclohexane with CH3 and a double bond.
- Identify the carbon atoms: Look for chiral centers.
- One of the carbon atoms in this compound is chiral because it is bonded to a CH3 group, a double bond, and two other different groups.
- Conclusion: Compound 3 shows optical isomerism.
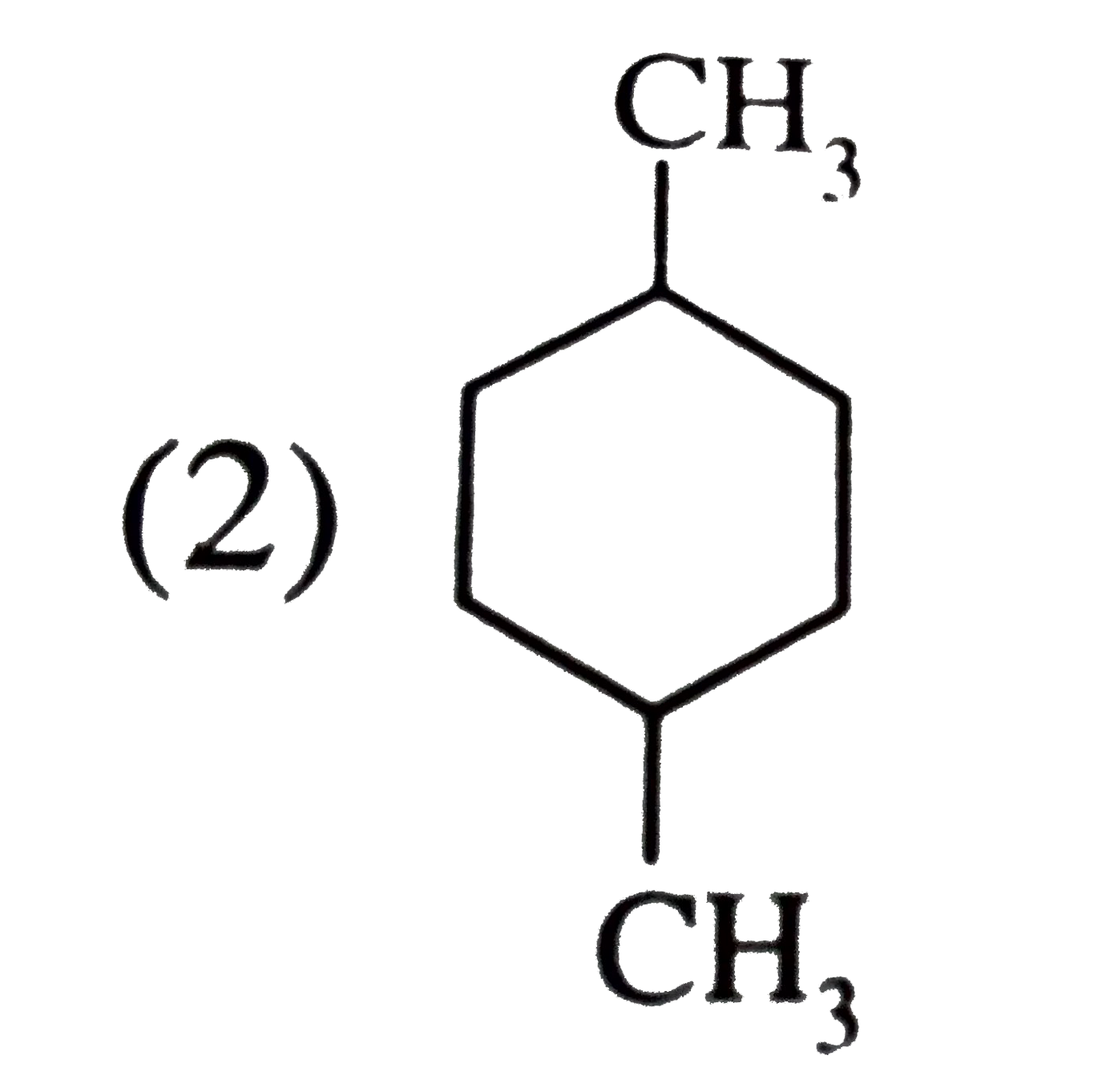
5. Analyze Compound 4:
- Since we have already determined the results for the first three compounds, we can conclude that compound 4 does not apply as it does not contain any additional chiral centers.
- Conclusion: Compound 4 does not show optical isomerism.
Final Answer:
The compounds that show optical isomerism are Compound 1 and Compound 3.
- Structure: CH3-CH(CH3)-CH2-CH(CH3)-CH3
- Identify the carbon atoms: Look for a carbon atom that has four different substituents.
- In this case, there are two carbon atoms that are chiral because they are bonded to different groups (two CH3 groups and two hydrogen atoms).
- Conclusion: Compound 1 shows optical isomerism.
- Structure: Cyclohexane with two CH3 groups.
- Identify the carbon atoms: Check if any carbon has four different substituents.
- Here, the carbons that have CH3 groups are not chiral because they are bonded to identical groups.
- Conclusion: Compound 2 does not show optical isomerism.
- Structure: Cyclohexane with CH3 and a double bond.
- Identify the carbon atoms: Look for chiral centers.
- One of the carbon atoms in this compound is chiral because it is bonded to a CH3 group, a double bond, and two other different groups.
- Conclusion: Compound 3 shows optical isomerism.
- Since we have already determined the results for the first three compounds, we can conclude that compound 4 does not apply as it does not contain any additional chiral centers.
- Conclusion: Compound 4 does not show optical isomerism.
The compounds that show optical isomerism are Compound 1 and Compound 3.
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
ज्ञान जांच
Which of the following compound shows optical isomerism ?
ACH3CH2CH3BCH2OHCHOHCH2OHCCH3CHOHC2H5DNone of theseWhich one of the following compounds will show geometrical isomerism ?
ACH3−CH=CH2BCH3−C∣CH3=CH2CCH3−C∣CH3=CHDDCH3=CH=CHDWhich of the following compound can show geometrical isomerism ?
A B
B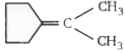 C
C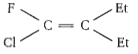 D
D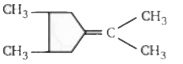
Which of the following compound show optical isomerism?
View SolutionWhich of the following compound show optical isomerism?
View SolutionWhich compounds will show optical isomerism
View SolutionWhich of the following compounds shows geometrical isomerism ?
View Solutionwhich of the following compound will show stereo isomerism?
View Solution
