Each phase of a material can exits only in certain regions of pressure and temperature . P−T phase diagrams, in which pressure is plotted versus temperature, show the regions corresponding to various phases and phase transformations . P−V diagrams, on the other hand , can be used to study pressure volume relationship at a constant temperature.
If the liquid and gaseous phases of a pure substances are heated together in a closed container, both the temperature and the vapor pressure will increase until a point is reached at which the two phases can no longer be distinguished from one another. The temperature and pressure at which this occurs are called the critical temperature and pressure. Exceeding either of these parameters, by itself ,will cause the gas/liguid phase transition to disappear. if the other variable is then changed as well, while the first variable is maintained above its critical point , a gradual transition will occur between the gaseous and liquid phases, with no clear boundary.(The liquid and solid phases, on the other hand , maintain a distinct boundary at all pressure above the triple point). Shown in figure is a combined P−T phase diagram for material A and B.
_E01_195_Q01.png)
If heat is added to solids A and B , each in a container that is open to the atmosphere :-
A will boil and B will melt
A will sublime and B will melt , then boil
A will melt and B will sublime
Both A and B will first melt, then boil
The correct Answer is:D
Since P' is constant = 1 atm , heat added will cause temperature rise. From the phase diagram, A will sublime while B will first melt and then boils.
টপার এই প্রশ্নগুলো সমাধান করেছে
- GEOMETRICAL OPTICSBOOK - ALLENCHAPTER - GEOMETRICAL OPTICSEXERCISE - EXERCISE - 04 (A)
47 Videos
- GEOMETRICAL OPTICSBOOK - ALLENCHAPTER - GEOMETRICAL OPTICSEXERCISE - EXERCISE - 04 (B)
15 Videos
- GEOMETRICAL OPTICSBOOK - ALLENCHAPTER - GEOMETRICAL OPTICSEXERCISE - ASSERTION-REASON
36 Videos
- CURRENT ELECTRICITYBOOK - ALLENCHAPTER - CURRENT ELECTRICITYEXERCISE - EX.II
66 Videos
- GRAVITATIONBOOK - ALLENCHAPTER - GRAVITATIONEXERCISE - EXERCISE 4
9 Videos
সম্পর্কিত প্রশ্ন
Each phase of a material can exits only in certain regions of pressure and temperature . P−T phase diagrams, in which pressure is plotted versus temperature, show the regions corresponding to various phases and phase transformations . P−V diagrams, on the other hand , can be used to study pressure volume relationship at a constant temperature.
If the liquid and gaseous phases of a pure substances are heated together in a closed container, both the temperature and the vapor pressure will increase until a point is reached at which the two phases can no longer be distinguished from one another. The temperature and pressure at which this occurs are called the critical temperature and pressure. Exceeding either of these parameters, by itself ,will cause the gas/liguid phase transition to disappear. if the other variable is then changed as well, while the first variable is maintained above its critical point , a gradual transition will occur between the gaseous and liquid phases, with no clear boundary.(The liquid and solid phases, on the other hand , maintain a distinct boundary at all pressure above the triple point). Shown in figure is a combined P−T phase diagram for material A and B.
_E01_196_Q01.png)
Which is true about the substance in figure?
View SolutionThe entropy change for a phase transformation is :
View Solution
জ্ঞান পরীক্ষা করুন
The true graphical representation of volume versus temperature relationship at constant pressure is :
A B
B C
C D
D
Which is the largest phase of M phase?
View SolutionDefine phase and phase difference.
View SolutionThe diagram below shows the change of phases of a substance on a temperature vs time graph on heating the substance at a constant rate.
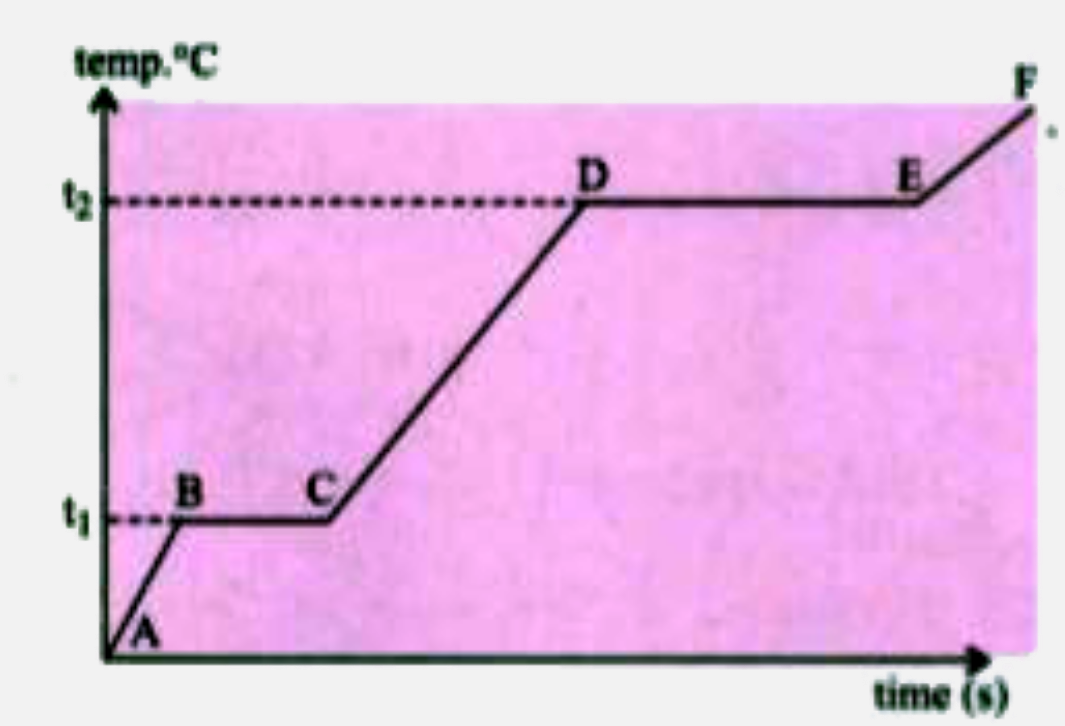
What is the boiling and melting point of the substance ?
View SolutionIn the graph plotted between vapour pressure (V.P.) and temperature (T),
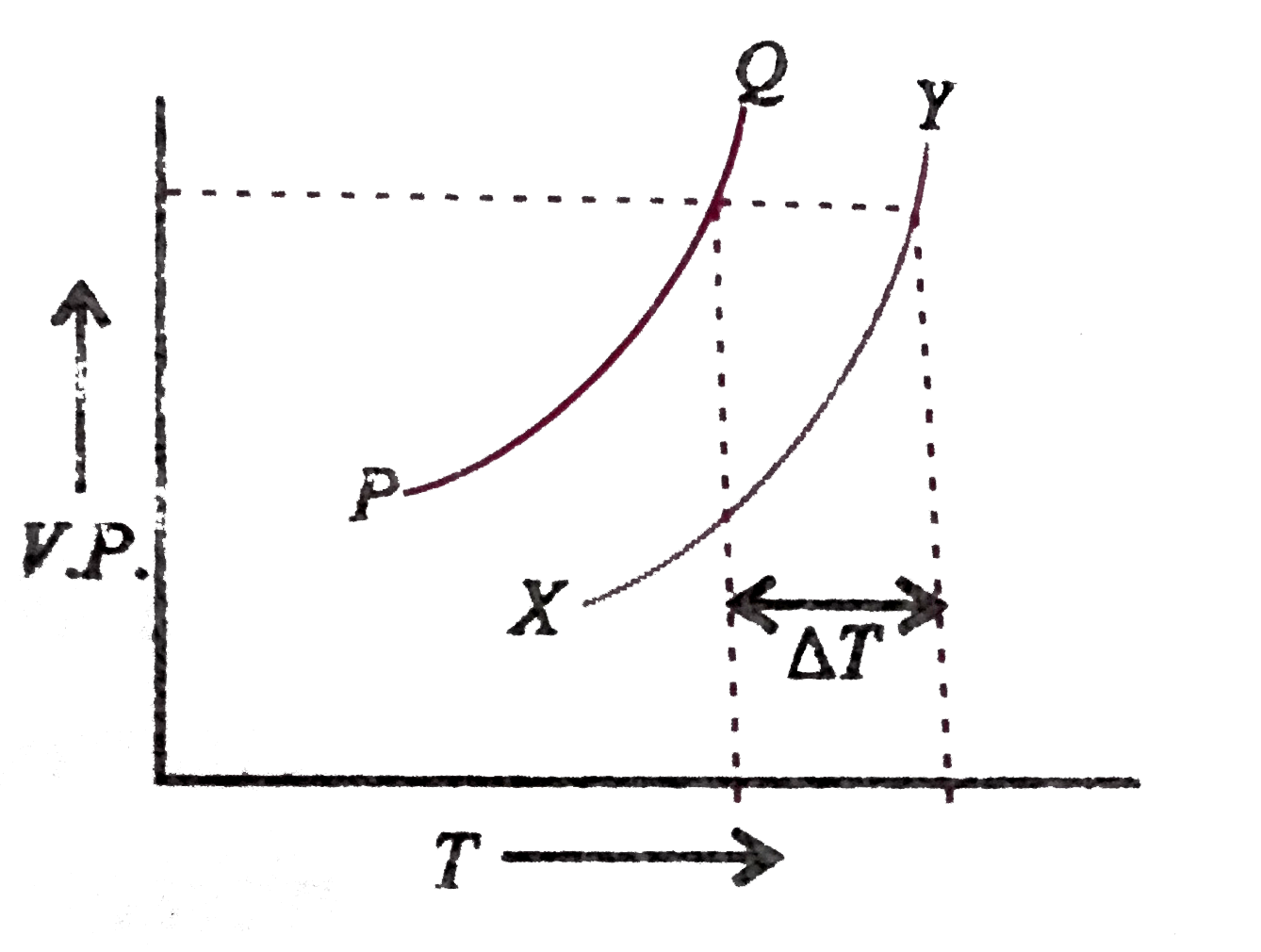
View SolutionVapour pressure diagram of some liquids plotted against temperature are shown below
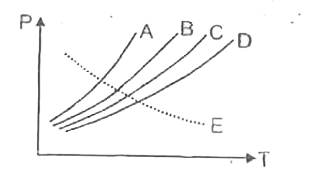
Most volatile liquid
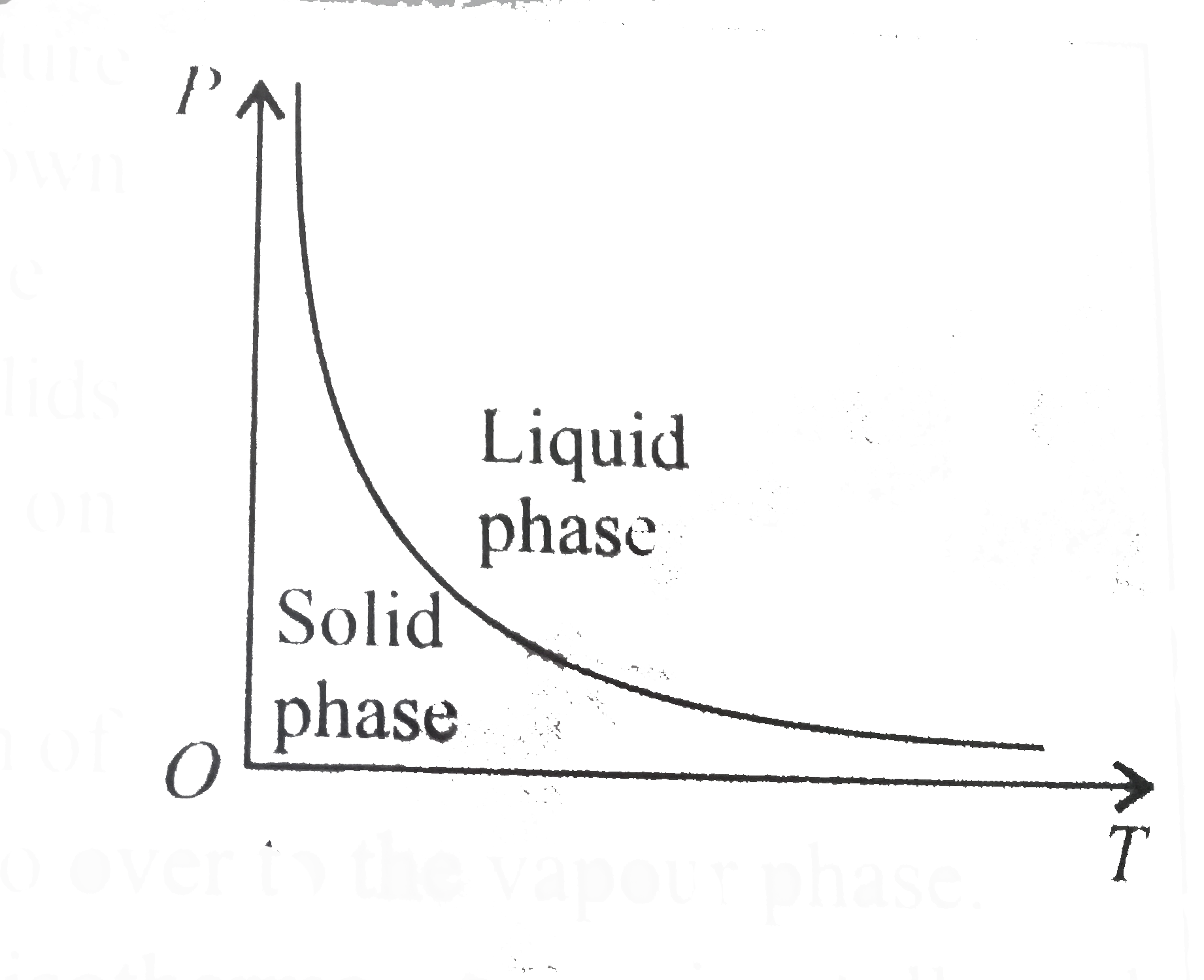
View SolutionThe pressure -temperature (P−T) phase diagram shown below corresponds to the
a. Curve of fusion of solids that expand on solidification.
b. Curve of sublimation of solids that directly go over to the vapour phase.
View SolutionWhy does the boundary between liquid phase and gaseous phase disappear on heating a liquid upto critical temperature in a closed vessel ? In this situation what will be the state of the substance ?
View Solution

