The ratio of work done by an ideal diatomic gas to the heat supplied by the gas in an isobaric process is
23
32
25
35
The correct Answer is:C
Step by step video solution for The ratio of work done by an ideal diatomic gas to the heat supplied by the gas in an isobaric process is by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE - 05 (B)
Explore 58 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE - 05 (B) (MCQ)
Explore 9 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE - 04 (B)
Explore 15 Videos
- CURRENT ELECTRICITYBook:ALLENChapter:CURRENT ELECTRICITYExercise:EX.II
Explore 66 Videos
- GRAVITATIONBook:ALLENChapter:GRAVITATIONExercise:EXERCISE 4
Explore 9 Videos
Similar Questions
A vessel contains a mixture of one mole of Oxygen and two moles of Nitrogen at 300 K. The ratio of the average kinetic energy per O2 molecule to that per N2 molecule is :
View SolutionLet →V,Vm s and Vp respectively, denote the mean speed, root mean square speed and most probable speed of the molecules in an ideal monatomic gas at absolute temperature T. The mass of a molecule is m. then :
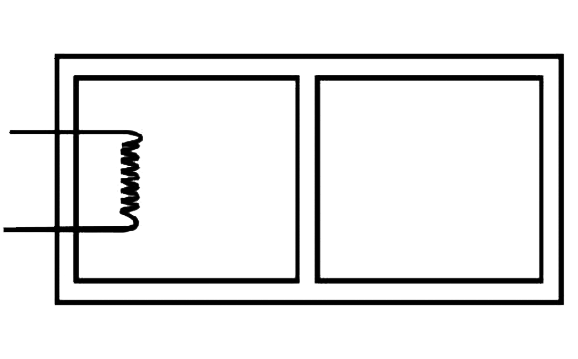
View SolutionThe rectangular box shown in Fig has partition which can slide without friction along the length of the box. Initially each of the two chambers of the box has one mole of a mono-atomic ideal gas (λ=5/3) at a pressure P0, volume V0 and temperature T0. The chamber on the left is slowly heated by an electric heater. The walls of the box and the lead wires of the heater is negligible. The gas in the left chamber expands pushing the partition until the final pressure in both chambers becomes 243P0/32. Determine (i) the final temperature of the gas in each chamber.
View SolutionIn the Bohr's model of hy drogen atom, the electron moves around the nucleus in a circular orbit of radius 5×10−11m. Its time period is 1.5×10−16s. The current associated with the electron motion is:-
View SolutionAssertion : The difference in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity of earth.
Reason : the value of acceleration due to gravity is minimum at the equator and maximum at the pole.
View SolutionIn isobaric process of ideal gas (f=5) work done by gas is equal of 10J. Then heat given to gas during process:
View SolutionThe ratio of work done by an ideal diatomic gas to the heat supplied by the gas in an isobatic process is
View SolutionIn an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is
View SolutionA diatomic gas done 80J work when expanded isobarically. Find the heat given to the gas during this process?
View SolutionIn an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is
View SolutionIn a thermodynamic process on an ideal diatomic gas, work done by the gas is η times. The heat supplied (η<1). The molar heat capacity of the gas for the process is
View SolutionIn an isobaric process of an ideal gas. The ratio of heat supplied annd work done by the system [i.e.,((Q)/(W))] is
View SolutionA sample of an ideal diatomic gas is heated at constant pressure. If an amount of 100 J of heat is supplied to the gas, find the work done by the gas.
View SolutionIn isobaric process of ideal gas (f=5) work done by gas is equal of 10J . Then heat given to gas during process:
View Solutionn moles of an ideal gas with constant volume heat capacity CV undergo an isobaric expansion by certain volume.The ratio of the work done in the process, to the heat supplied is :
View SolutionIn a thermodynamic process on an ideal diatomic gas, work done by the gas is η times the heat supplied (η<1). The molar heat capacity of the gas for the process is
View Solution
