In each of the following pair of compounds,w hich is more basic in aqueous solution?
Give an explanation for your choice:
(a). CH3NH2 or CF3NH2
(b). 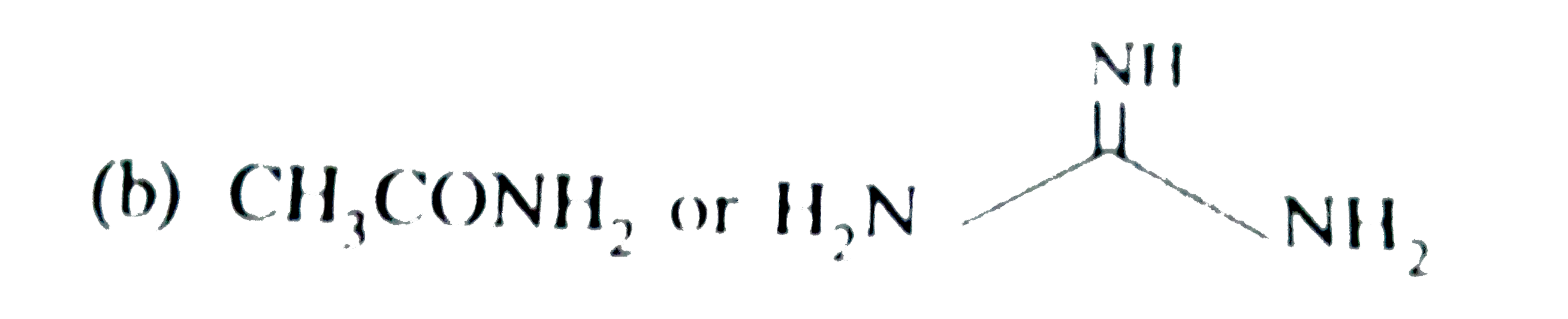 ltBrgt (c). CH3CH2CH2NH2 or CH3CN
ltBrgt (c). CH3CH2CH2NH2 or CH3CN
(d). C6H5N(CH3)2 or 2,6-dimethyl-N-N-dimethylaniline.
The correct Answer is:(a) I, (b) ii, (c) I, (d) ii
Step by step video solution for In each of the following pair of compounds,w hich is more basic in aqueous solution? Give an explanation for your choice: (a). CH_(3)NH_(2) or CF_(3)NH_(2) (b). ltBrgt (c). CH_(3)CH_(2)CH_(2)NH_(2) or CH_(3)CN (d). C_(6)H_(5)N(CH_(3))_(2) or 2,6-dimethyl-N-N-dimethylaniline. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ACIDIC STRENGTH & BASIC STRENGTHBook:ALLENChapter:ACIDIC STRENGTH & BASIC STRENGTHExercise:Exercise III
Explore 14 Videos
- ACIDIC STRENGTH & BASIC STRENGTHBook:ALLENChapter:ACIDIC STRENGTH & BASIC STRENGTHExercise:Exercise IV
Explore 30 Videos
- ACIDIC STRENGTH & BASIC STRENGTHBook:ALLENChapter:ACIDIC STRENGTH & BASIC STRENGTHExercise:Exercise V
Explore 16 Videos
- CONCENTRATION TERMSBook:ALLENChapter: CONCENTRATION TERMSExercise:Exercise Jee-Advance
Explore 3 Videos
- AIIMS 2019Book:ALLENChapter:AIIMS 2019Exercise:CHEMISTRY
Explore 40 Videos
Similar Questions
CH3CH2NH2 is soluble in :-
View SolutionWhich of the following compounds will not exist as resonance hybrid. Give reason for your answer.
(a) CH3OH
(b) R−CONH2
(c) CH3CH=CHCH2NH2
View SolutionAniline in a set of reactions yielded a product B
(a)C6H5CH2NH2
(b)C6H5NHCH2CH3
(c)C6H5NHOH
(d)C6H5CH2OH
View SolutionWrite chemical equations for the following conversions:
(i)CH3−CH2−ClintoCH3−CH2CH2−NH2
(ii)C6H5−CH2−ClintoC6H5−CH2−NH2
View SolutionWhich is the correct order of increasing basicity?
(A) CH3CH2CH3<CH3CH2SH<CH3CH2OH< CH3CH2NH2
(B)CH3CH2CH3<CH3CH2OH<CH3CH2SH< CH3CH2NH2
(C) CH3CH2NH2<CH3CH2SH<CH3CH2OH< CH3CH2CH3
(D)CH3CH2CH3<CH3CH2OH<CH3CH2NH2< CH3CH2SH
View SolutionArrange the following anilines in decreasing order of basicity
1. C6H5NH2
2. o−CH3C6H4NH2
3. m−CH3C6H4NH2
4. p−CH3C6H4NH2
View SolutionWrite IUPAC name of CH3−CH2−NH2
View SolutionWhich of the following pairs is expected to exhibit H-bonding
(a) CH3CH2OH and CH3OCH3
(b) CH3NH2 and CH3SH
(c ) CH3OH and (CH3)3N .
View SolutionDistinction between pairs of compounds
Ethylamine (CH3CH2NH2) and diethylamine (CH3CH2NHCH2CH3)
View SolutionDistinction between pairs of compounds
Ethylamine (CH3CH2NH2) and diethylamine (CH3CH2NHCH2CH3)
View Solution
