Species acting as both Bronsted acid and base is:
NH3
OH−
HSOΘ4
1 and 3 both
The correct Answer is:D
Step by step video solution for Species acting as both Bronsted acid and base is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ACIDIC STRENGTH & BASIC STRENGTHBOOK - ALLENCHAPTER - ACIDIC STRENGTH & BASIC STRENGTHEXERCISE - Exercise V
16 Videos
- ACIDIC STRENGTH & BASIC STRENGTHBOOK - ALLENCHAPTER - ACIDIC STRENGTH & BASIC STRENGTHEXERCISE - Exercise III
14 Videos
- CONCENTRATION TERMSBOOK - ALLENCHAPTER - CONCENTRATION TERMSEXERCISE - Exercise Jee-Advance
3 Videos
- AIIMS 2019BOOK - ALLENCHAPTER - AIIMS 2019EXERCISE - CHEMISTRY
40 Videos
Similar Questions
In the following compounds
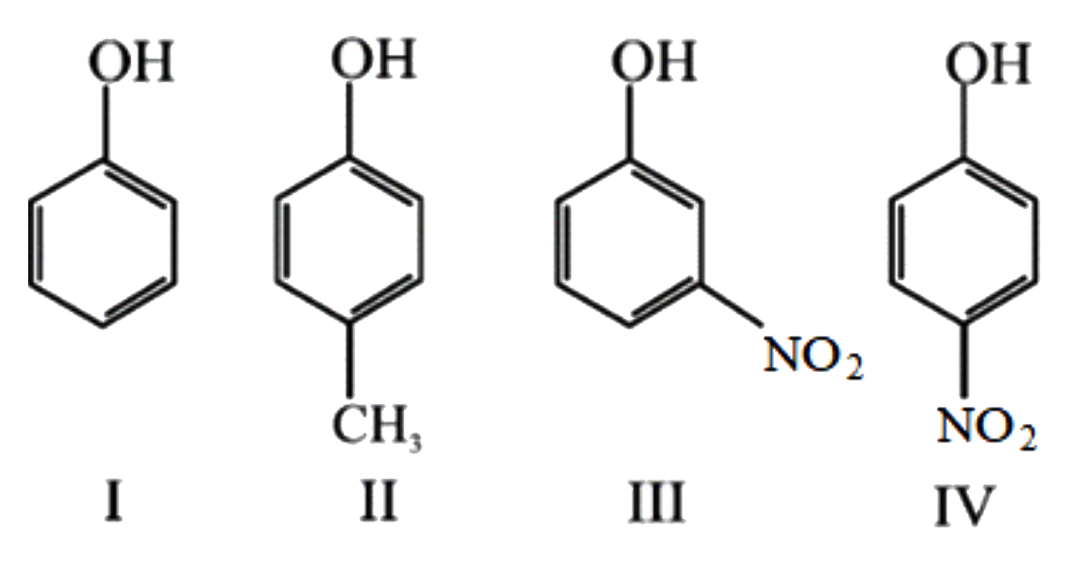
The order of acidity is
View SolutionIn given reaction gas liberated is/are
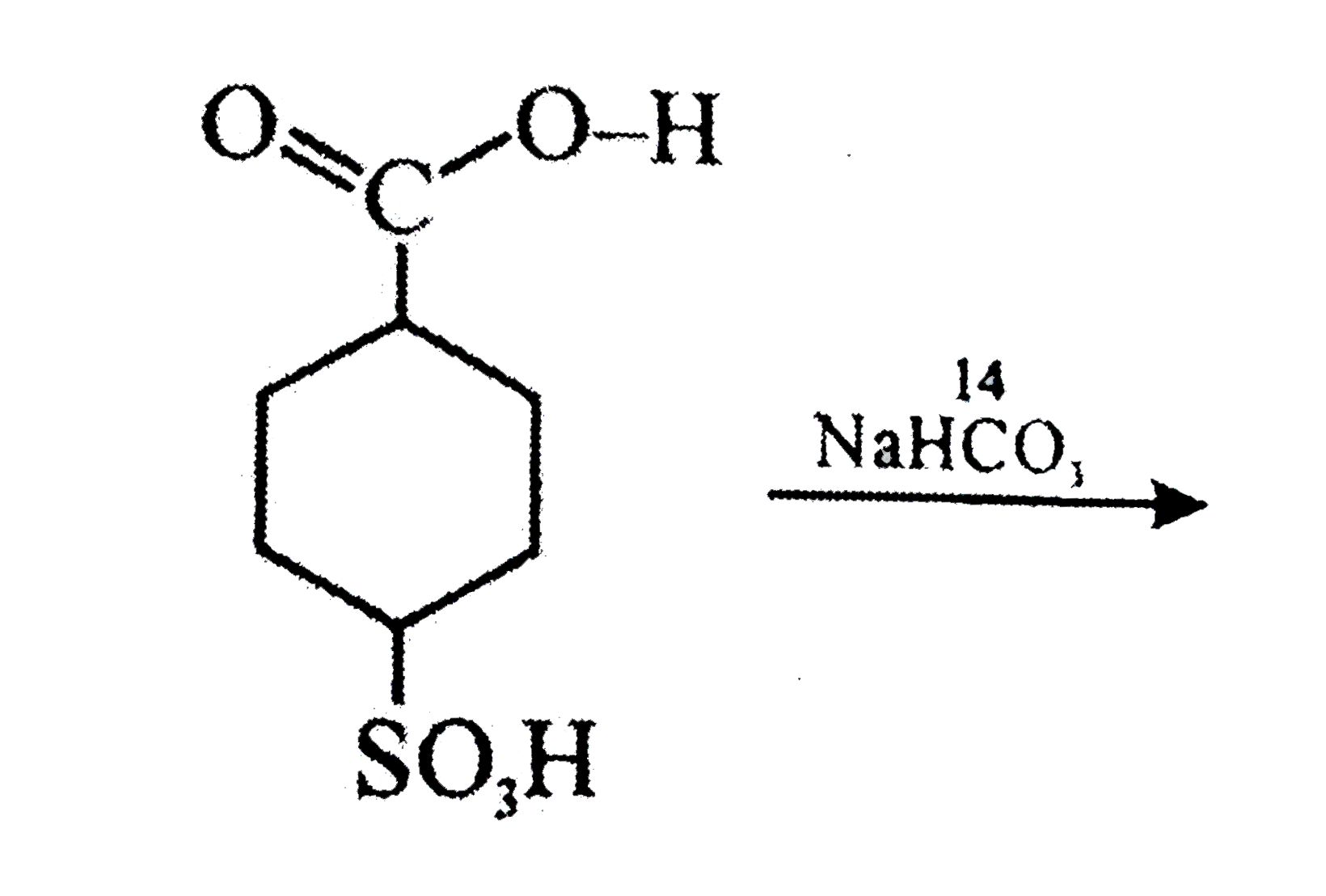
View SolutionCalculate the molarity of water if its density is 1000kgm−3
View SolutionSelect the correct statements
View SolutionWhich of the following can act both as a Bronsted acid and a Bronsted base?
View SolutionSpecies acting as both Bronsted acid and base is:
View SolutionWhich of the following species acts as Bronsted base but not as acid
View SolutionWhich one of the following species cannot act as both Bronsted acid and base ?
View SolutionSpecies acting both as Bronsted acid base is
View SolutionWrite four species which act both as Bronsted acid as well as base. Write their corresponding conjugate acids and bases.
View SolutionWhich one of the following species cannot act as both Bronsted acid and base ?
View SolutionWhich one of the following species acts as both Bronsted acid and base ?
View SolutionWhich one of the following species acts as both Bronsted acid and base ?
View SolutionThe species that can act both as Bronsted acid and as Bronsted base in water are-
View SolutionSpecies acting both as Bronsted acid and base is
View SolutionWhich of the following species acts as Bronsted base but not as acid ?
View Solution

