10mL of 2(M) NaOH solution is added to 200mL of 0.5 (M) of NaOH solution. What is the final concentration?
0.57M
5.7M
11.4M
1.14M
The correct Answer is:A
To find the final concentration of the NaOH solution after mixing two different concentrations, we can follow these steps:
1. Identify the given values:
- Volume of the first solution (V1) = 10 mL
- Molarity of the first solution (M1) = 2 M
- Volume of the second solution (V2) = 200 mL
- Molarity of the second solution (M2) = 0.5 M
2. Convert volumes to liters:
- V1 = 10 mL = 10/1000 L = 0.01 L
- V2 = 200 mL = 200/1000 L = 0.2 L
3. Calculate the total volume (V_final):
- V_final = V1 + V2 = 0.01 L + 0.2 L = 0.21 L
4. Calculate the total moles of NaOH from both solutions:
- Moles from the first solution = M1 × V1 = 2 M × 0.01 L = 0.02 moles
- Moles from the second solution = M2 × V2 = 0.5 M × 0.2 L = 0.1 moles
5. Calculate the total moles of NaOH:
- Total moles = Moles from first solution + Moles from second solution
- Total moles = 0.02 moles + 0.1 moles = 0.12 moles
6. Calculate the final concentration (M_final):
- M_final = Total moles / V_final
- M_final = 0.12 moles / 0.21 L = 0.5714 M
7. Round to two decimal places:
- Final concentration ≈ 0.57 M
Final Answer:
The final concentration of the mixed NaOH solution is approximately 0.57 M.
- Volume of the first solution (V1) = 10 mL
- Molarity of the first solution (M1) = 2 M
- Volume of the second solution (V2) = 200 mL
- Molarity of the second solution (M2) = 0.5 M
- V1 = 10 mL = 10/1000 L = 0.01 L
- V2 = 200 mL = 200/1000 L = 0.2 L
- V_final = V1 + V2 = 0.01 L + 0.2 L = 0.21 L
- Moles from the first solution = M1 × V1 = 2 M × 0.01 L = 0.02 moles
- Moles from the second solution = M2 × V2 = 0.5 M × 0.2 L = 0.1 moles
- Total moles = Moles from first solution + Moles from second solution
- Total moles = 0.02 moles + 0.1 moles = 0.12 moles
- M_final = Total moles / V_final
- M_final = 0.12 moles / 0.21 L = 0.5714 M
- Final concentration ≈ 0.57 M
The final concentration of the mixed NaOH solution is approximately 0.57 M.
टॉपर्स ने हल किए ये सवाल
- CONCENTRATION TERMSBOOK - ALLENCHAPTER - CONCENTRATION TERMSEXERCISE - Exercise Jee-Advance
3 Videos
- CONCENTRATION TERMSBOOK - ALLENCHAPTER - CONCENTRATION TERMSEXERCISE - Exercise O-II
23 Videos
- ACIDIC STRENGTH & BASIC STRENGTHBOOK - ALLENCHAPTER - ACIDIC STRENGTH & BASIC STRENGTHEXERCISE - Exercise V
16 Videos
समान प्रश्न
Calculate the molarity of water if its density is 1000kgm−3
View SolutionStatement-1 : Molality of pure ethanol is lesser than pure water
Statement-2 : As density of ethanol is lesser than density of water
[Given : dethanol=0.789gm/ml,dwater=1gm/ml]
View SolutionIn the following compounds
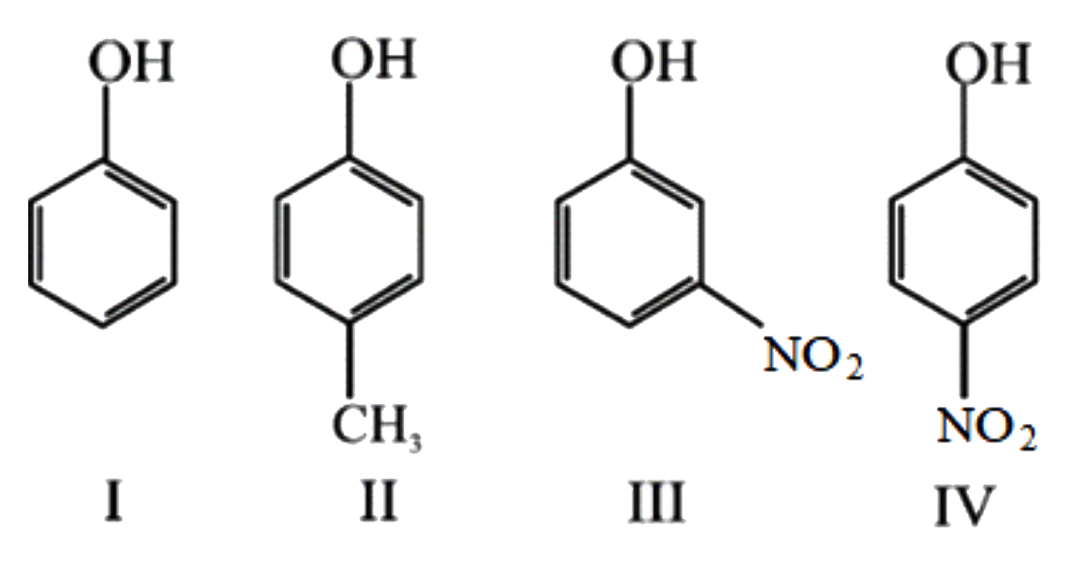
The order of acidity is
View Solution2.5 litre of 1 M NaOH solution are mixed with another 3 litre of 0.5 M NaOH solution Then the molarity of the resulting
View SolutionA 25.00mL sample of 0.1050 M H_(2)SO_(4) is titrated with a NaOH solution of unknown concentration. The phenophthalein endpoint was reached when 17.23 mL of the NaOH solution had been added. What is the concentration of the NaOH?
View SolutionIf 40 gm of NaOH is added to 2 litre solution of 4 M NaOH, find the molality of final solution (density of solution=0.68 g/ml).
View SolutionWhat volume of water is to be a added to 100cm3 of 0.5 M NaOH solution to make it 0.1 M solution?
View SolutionAre 0.5 mole of NaOH and 0.5 M of NaOH solution same ?
View SolutionWhat volume of water should be added to 300 mL of 0.5 M NaOH solution so as to get a solution of 0.2 M ?
View Solution2.5 litres of 1M NaOH solution is mixed with 3.0 litres of 0.5 M NaOH solution. The molarity of resulting solution is :
View Solution2.5 litre of 1M NaOH solution is mixed with a 3.0 litres of 0.5 M NaOH solution. The molarity of the resulting solution is :
View SolutionWhat volume of water is to be added to 100 cm^3 of 0.5 M NaOH solution to make it 0.1 M solution ?
View SolutionTo a 4 L of 0.2 M solution of NaOH, 2L of 0.5 M NaOH are added. The molarity of the resulting solution is :
View SolutionTo a 4 L of 0.2 M solution of NaOH, 2L of 0.5 M NaOH are added. The molarity of the resulting solution is :
View Solution0.1 M विलयन को बनाने के लिए 0.5 M NaOH विलयन के 100cm3 विलयन में जल की कितनी मात्रा को मिलाना चाहिए?
View Solution10mL, 0.1 M NaOH, 10mL of 0.05M H2SO4 युक्त विलयन की pH होगा :-
View Solution

