A gaseous mixture of H2andNH3 gas contains 68 mass % of
NH3 . The vapour density of the mixture is-
To find the vapor density of a gaseous mixture of H2 and NH3 that contains 68 mass % of NH3, we can follow these steps:
Step 1: Determine the Masses of the Gases
Given that the mixture contains 68 mass % of NH3, we can assume a total mass of the mixture to be 100 grams for simplicity.
- Mass of NH3 = 68 grams
- Mass of H2 = 100 grams - 68 grams = 32 grams
Step 2: Calculate the Number of Moles of Each Gas
Next, we need to calculate the number of moles of NH3 and H2.
- Molar mass of NH3 = 14 (N) + 3 × 1 (H) = 17 g/mol
- Moles of NH3 = 68 g17 g/mol=4 moles
- Molar mass of H2 = 2 × 1 (H) = 2 g/mol
- Moles of H2 = 32 g2 g/mol=16 moles
Step 3: Calculate the Total Number of Moles
Now, we can find the total number of moles in the mixture.
- Total moles = Moles of NH3 + Moles of H2
- Total moles = 4+16=20 moles
Step 4: Calculate the Average Molecular Mass of the Mixture
The average molecular mass (M) of the mixture can be calculated using the formula:
M=Total mass of the mixtureTotal number of moles
- Total mass of the mixture = 100 grams
- Average molecular mass M=100 g20 moles=5 g/mol
Step 5: Calculate the Vapor Density
Vapor density (VD) is defined as half of the average molecular mass.
VD=M2
- Vapor density VD=5 g/mol2=2.5 g/L
Final Answer
The vapor density of the mixture is 2.5 g/L.
---
Given that the mixture contains 68 mass % of NH3, we can assume a total mass of the mixture to be 100 grams for simplicity.
- Mass of H2 = 100 grams - 68 grams = 32 grams
Next, we need to calculate the number of moles of NH3 and H2.
- Moles of NH3 = 68 g17 g/mol=4 moles
- Moles of H2 = 32 g2 g/mol=16 moles
Now, we can find the total number of moles in the mixture.
- Total moles = 4+16=20 moles
The average molecular mass (M) of the mixture can be calculated using the formula:
- Average molecular mass M=100 g20 moles=5 g/mol
Vapor density (VD) is defined as half of the average molecular mass.
The vapor density of the mixture is 2.5 g/L.
Topper's Solved these Questions
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - Some Solved Examples
9 Videos
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - Basic Exercise
29 Videos
- METALLURGYBOOK - ALLENCHAPTER - METALLURGYEXERCISE - EXERCISE-05 [B]
26 Videos
- p-Block Element BOOK - ALLENCHAPTER - p-Block Element EXERCISE - All Questions
20 Videos
Similar Questions
When the same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volume of hydrogen evolved is :
View Solution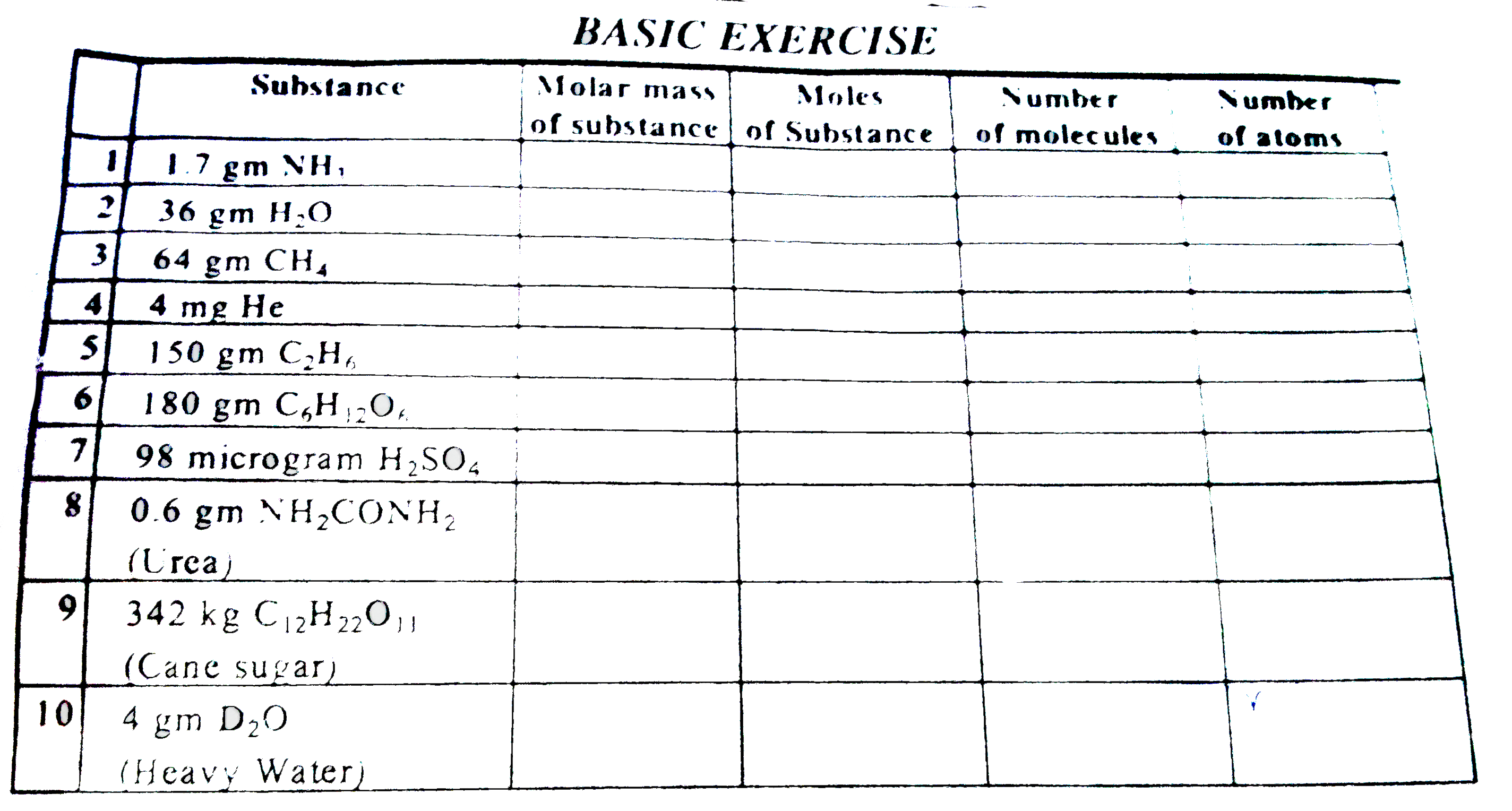
A box contains 12×1022 number of oxygen atoms. Find moles of O-atoms? (NA=6×1023)
View SolutionWhen the ore haematite is burnt in air with coke around 2000K along with lime, the process not only produces steel but also produces a silicate slag that is useful in making building materials such as cement. Discuss the same and show through balanced chemical equation.
View SolutionThe main factor responsible for weak acidic nature of BF3 is :-
View SolutionIf 340g of a mixture of N2 and H2 in the correct ratio gas a 20% yield of NH3. The mass produced would be:
View SolutionA gaseous mixture of H2 and CO2 gas contains 66 mass % of CO2 . The vapour density of the mixture is :
View SolutionThe vapour density of a aseous mixture of non-reactive gases 'A' and 'B' is 40. if the molar mass of gas 'A' is 20 g/mol and the mixture contains the gases in 2:3 volume ratio, then the molar mass of gas 'B' is
View SolutionOne litre of a gaseous mixture of two gases mixture of two gases effuses in 311 seconds while 2 litre of oxygen takes 20 minutes. The vapour density of gaseous mixture containing CH4 and H2 is:
View SolutionA gaseous mixture of H_2 and CO_2 gases contains 66 mass % of CO_2 . The vapour density of the mixture is :
View Solution81 gm mixture of mgCO_(3)(s) and NH_(3)CO_(3)(s) is heated to constant mass. If vapour density of gaseous mixture evolved was found to be (61)/(4) then Given that: MgCo_(3)(s) rarr MgO_(s) + CO_(2)(g) NH_(2)COONH_(4)(s) rarr 2NH_(3)(g)+CO_(2)(g) Mole % of MgCo_(3) in original sample:
View Solution34 gm of a mixture containing N_(2) and H_(2) in 1:3 by mole is partially coverted into NH_(3) . Calculate the molar mass of the mixture (containing remaining N_(2),H_(2) and NH_(3) formed) after reaction if it has been found that the NH_(3) formed required 0.5 moles of H_(3)PO_(4) for complete neutralization 3NH_(3)+H_(2)PO_(4)rarr(NH_(4))_(3)PO_(4) Write nearest integral value.
View SolutionAmmonia gas is passed into water, yielding a solution of density 0.93g/cm3 and containing 18.6%NH3 by weight. The mass of NH3 per cc of the solution is :
View SolutionA gaseous mixture contains 336cm3 of H2 and 224cm3 of He at STP. The mixture shows a pressure of 2 atm when it is kept in a container at 27∘C. Calculate the volume of the gas?
View SolutionThe vapour density of a mixture of gas A (Molecular mass = 40) and gas B (Molecular mass = 80) is 25. Then mole % of gas B in the mixture would be
View SolutionThe vapour density of a mixture containing N2(g)andO2(g) is 14.4. The percentage of N2 in the mixture is :
View SolutionA gaseous mixture of H2andCO2 gases contains 66 mass % of CO2. The vapour density of the mixture is :
View Solution

