An iodized salt contains 0.5% of NaI. A person consumes 3 gm of salt everyday. The number of iodide ions going into his body everyday is (I = 127 )
10−4
6.02×10−4
6.02×1019
6.02×1023
The correct Answer is:C
To solve the problem step by step, we will follow the given information and apply the necessary calculations.
Step 1: Calculate the weight of NaI in 3 grams of salt
The iodized salt contains 0.5% of NaI. This means that in 100 grams of salt, there are 0.5 grams of NaI.
To find the weight of NaI in 3 grams of salt:
Weight of NaI=0.5100×3=0.015 grams=151000 grams
Step 2: Calculate the molar mass of NaI
The molar mass of NaI can be calculated by adding the atomic masses of sodium (Na) and iodine (I).
- Atomic mass of Na = 23 g/mol
- Atomic mass of I = 127 g/mol
Thus, the molar mass of NaI is:
Molar mass of NaI=23+127=150 g/mol
Step 3: Calculate the number of moles of NaI
Using the weight of NaI calculated in Step 1 and the molar mass from Step 2, we can find the number of moles of NaI:
Number of moles of NaI=Weight of NaIMolar mass of NaI=15×10−3 g150 g/mol=10−4 moles
Step 4: Determine the number of iodide ions (I⁻)
Since NaI dissociates completely in solution, the number of moles of iodide ions (I⁻) will be equal to the number of moles of NaI:
Moles of I⁻=10−4 moles
Step 5: Calculate the number of iodide ions
To find the total number of iodide ions, we multiply the number of moles of iodide ions by Avogadro's number (approximately 6.022×1023 mol⁻¹):
Number of iodide ions=Moles of I⁻×Avogadro's number=10−4×6.022×1023≈6.022×1019 ions
Final Answer
The number of iodide ions going into the person's body every day is approximately 6.022×1019.
---
The iodized salt contains 0.5% of NaI. This means that in 100 grams of salt, there are 0.5 grams of NaI.
Weight of NaI=0.5100×3=0.015 grams=151000 grams
The molar mass of NaI can be calculated by adding the atomic masses of sodium (Na) and iodine (I).
- Atomic mass of Na = 23 g/mol
- Atomic mass of I = 127 g/mol
Molar mass of NaI=23+127=150 g/mol
Using the weight of NaI calculated in Step 1 and the molar mass from Step 2, we can find the number of moles of NaI:
Number of moles of NaI=Weight of NaIMolar mass of NaI=15×10−3 g150 g/mol=10−4 moles
Since NaI dissociates completely in solution, the number of moles of iodide ions (I⁻) will be equal to the number of moles of NaI:
Moles of I⁻=10−4 moles
To find the total number of iodide ions, we multiply the number of moles of iodide ions by Avogadro's number (approximately 6.022×1023 mol⁻¹):
Number of iodide ions=Moles of I⁻×Avogadro's number=10−4×6.022×1023≈6.022×1019 ions
The number of iodide ions going into the person's body every day is approximately 6.022×1019.
টপার এই প্রশ্নগুলো সমাধান করেছে
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:O-II
Explore 11 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:O-II (Assertion Reason:)
Explore 2 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:S-II
Explore 18 Videos
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE-05 [B]
Explore 26 Videos
- p-Block Element Book:ALLENChapter:p-Block Element Exercise:All Questions
Explore 20 Videos
সম্পর্কিত প্রশ্ন
A solution containing 0.85g of ZnCl2 in 125.0g of water freezes at −0.23∘C. The apparent degree of dissociation of the salt is:
(kf for water =1.86Kkgmol−1, atomic mass, Zn=65.3 and Cl=35.5)
View SolutionThe atomic number of chromium is 24 Its electronic coefigueration in ground state in 1s22s22p63s24s13d5 .Chromium atom by using 3 electron from Cr3+ .A chromium atom contain 17% morte neutron than the proton. Now answer the following questions
The number of unpaired electron in Cr3+ion is
View Solution
জ্ঞান পরীক্ষা করুন
Body mass index (BMI)is a comparison of a person's body mass to his or her height. A high BMI can be an indicator of high body fat, which can lead to health problems. According to the American Heart Association, an adult is underweight if his or her BmI is less than 18.5, or overwieght if it is greater than or equal to 25.0. Which of the following number lines could be used to model a healthy BMI range for an adult?
A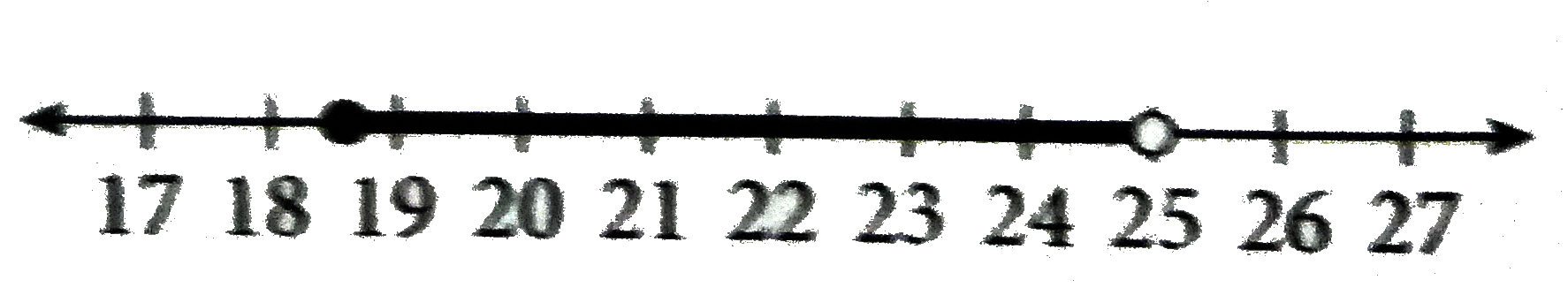 B
B C
C D
D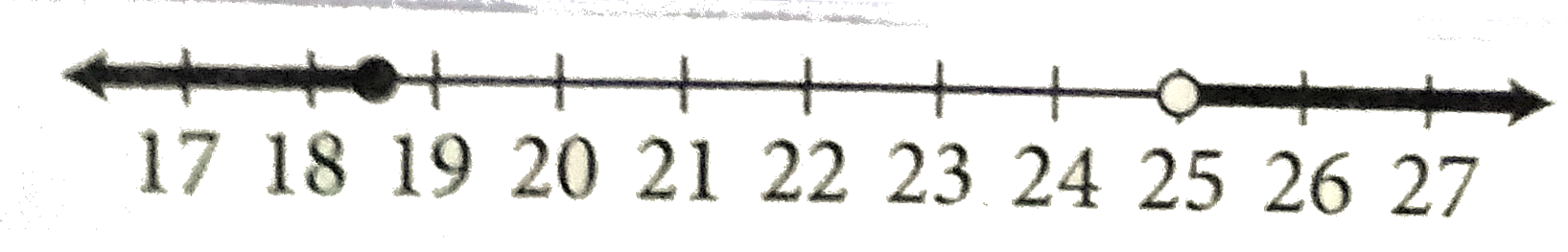
The atomic number of chromium is 24 Its electronic coefigueration in ground state in 1s22s22p63s24s13d5 .Chromium atom by using 3 electron from Cr2+ .A chromium atom contain 17% morte neutron than the proton. Now answer the following questions
The number of occupied sub- shell in Cr3+ ion is
View Solutioni. 0.21 gm of but-3-yn-2-ol is treated with excess of C2H5MgBr at standard condition. The volume of gas evolved is:
(a) 134.4 ml, (b) 146.4 ml
(c) 67.2 ml, (d) 73.2 ml
ii. 0.46 gm of a compound with molecular mass of 92 gm gave 336 ml of a gas at STP when treated with excess of CH3MgI. The number of moles in the compound is:
(a) 0.1, (b) 2
(c) 3, (d) 4
iii. The treatement of CH3OH with CH3Mgl releases 1.04ml of a gas at STP. The mass of CH3OH added is:
(a) 1.49 mg,
(b) 2.98 mg
(c) 3.71 mg, (d) 4047 mg
iv. The addition of 4.12 mg of an unknown alcohol, ROH, to CH3Mgl releases 1.56 ml of a gas at STP. The molar mass of alcohol is:
(a) 32 gm mol−1 , (b) 46 gm mol−1
(c) 59gmmol−1 , (d) 74 gm mol−1
v. Teh sample of 1.79 mg of a compound of molar mass 90 gm mol−1 when treated with CH3Mgl releases 1.34 mol of a gas at STP. The number of moles of active hydrogen in the molecule is:
(a) 1 , (b) 2
(c) 3 , (d) 4
View Solution100 ml aqueous solution (density53gm/ml) contains 40% by weight NaOH. The number of molecules of NaOH dissolved in the above solution is :
(UseNA=6×1023)
View SolutionA current of 5.0 A flows for 4.0 h through an electrolytic cell containing a molten slat of metal M. This result in deposition of 0.25 mol of the metal M at the cathode. The oxidation state of M in the molten salt is +x. The value of 'x' is (1 Faraday = 96000 C mol−1) 3
View SolutionA small quantity of solution containing Na24 radio nuclide (half−life=15h) of activity 1.0microcurie is injected into the blood of a person. A sample of the blood of volume 1cm3 taken after 5h shows an activity of 296 disintegrations per minute. Determine the total volume of the blood in the body of the person. Assume that the radioactive solution mixes uniformly in the blood of person.
(1 curie =3.7×1010 disintegrations per second)
View SolutionA small quantity of solution containing Na24 radio nuclide (half−life=15h) of activity 1.0microcurie is injected into the blood of a person. A sample of the blood of volume 1cm3 taken after 5h shows an activity of 296 disintegrations per minute. Determine the total volume of the blood in the body of the person. Assume that the radioactive solution mixes uniformly in the blood of person.
(1 curie =3.7×1010 disintegrations per second)
View SolutionA solution of 0.2 g of a compound containing Cu2+andC2O2−4 ions on titration with 0.02 M KMnO4 in presence of H2SO4 consumes 22.6 mL of the oxidant. The resultant solution is neutralized with Na2CO3 acidified with dil. acetic acid and treated with excess KI. The liberated iodine requires 11.3 mL of 0.5 M Na2S2O3 solution for complete reduction. Find out the mole ratio of Cu2+toC2O2−4 in the compound. Write down the balanced redox reactions involved in the above titrations.
View Solution
