Assertion : Rate of reaction
CD4+Cl2→ Slow CH4+Cl2→Fast.
Reason : Due to isotopic effect.
If both Assertion & Reason are True & the Reason is a correct explanation of the Assertion.
If both Assertion & Reason are True but Reason is not a correct explanation of the Assertion.
If Assertion is True but the Reason is False.
If both Assertion & Reason are False.
The correct Answer is:A
Step-by-Step Solution:
1. Understanding the Reactions:
- The two reactions given are:
- CD4+Cl2→Slow
- CH4+Cl2→Fast
2. Identifying the Assertion:
- The assertion states that the reaction of CD4 with Cl2 is slow, while the reaction of CH4 with Cl2 is fast.
3. Identifying the Reason:
- The reason provided is that this difference in reaction rates is due to the isotopic effect.
4. Explaining the Isotopic Effect:
- The isotopic effect refers to the change in reaction rates due to the presence of isotopes. In this case, deuterium (D) is heavier than hydrogen (H).
- The bond between carbon and deuterium (C-D) is stronger and less vibrationally active compared to the bond between carbon and hydrogen (C-H).
5. Analyzing the Strength of Bonds:
- The C-D bond is stronger than the C-H bond because deuterium is heavier, leading to less vibrational motion.
- As a result, CD4 is more stable and reacts slower with Cl2.
6. Comparing Reaction Rates:
- In contrast, CH4 has a weaker C-H bond, which vibrates more due to the lighter mass of hydrogen. This increased vibrational energy makes CH4 less stable and more reactive, leading to a faster reaction with Cl2.
7. Conclusion:
- Both the assertion and the reason are true, and the reason correctly explains the assertion. Therefore, the correct answer is that both the assertion and reason are true, and the reason is a correct explanation of the assertion.
Final Answer:
Both the assertion and the reason are true, and the reason is a correct explanation of the assertion.
- The two reactions given are:
- CD4+Cl2→Slow
- CH4+Cl2→Fast
- The assertion states that the reaction of CD4 with Cl2 is slow, while the reaction of CH4 with Cl2 is fast.
- The reason provided is that this difference in reaction rates is due to the isotopic effect.
- The isotopic effect refers to the change in reaction rates due to the presence of isotopes. In this case, deuterium (D) is heavier than hydrogen (H).
- The bond between carbon and deuterium (C-D) is stronger and less vibrationally active compared to the bond between carbon and hydrogen (C-H).
- The C-D bond is stronger than the C-H bond because deuterium is heavier, leading to less vibrational motion.
- As a result, CD4 is more stable and reacts slower with Cl2.
- In contrast, CH4 has a weaker C-H bond, which vibrates more due to the lighter mass of hydrogen. This increased vibrational energy makes CH4 less stable and more reactive, leading to a faster reaction with Cl2.
- Both the assertion and the reason are true, and the reason correctly explains the assertion. Therefore, the correct answer is that both the assertion and reason are true, and the reason is a correct explanation of the assertion.
Both the assertion and the reason are true, and the reason is a correct explanation of the assertion.
Topper's Solved these Questions
Similar Questions
Calgon is an industrial name given to
View Solution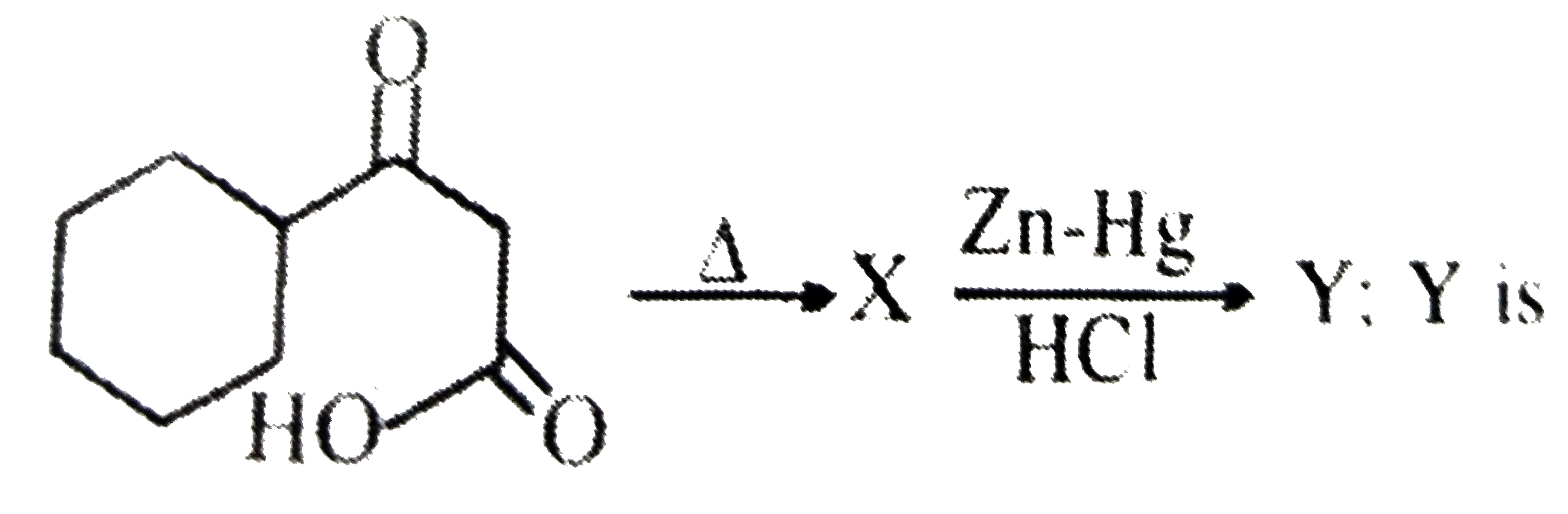
View SolutionIn order to refine "copper" it is melted in a furnace and it is stirred with green logs of wood. The purpose is :-
View SolutionMechanism of the reaction 2NO + Cl_(2) rarr 2NOCl may be written as 2NO overset(k)(hArr) (NO)_(2) ……….(fast) (NO)_(2) + Cl_(2) overset(k)rarr 2NOCl ……….(slow) Rate equation would be
View SolutionConsider the reaction,
Cl2(aq)+H2S(aq)→S(s)+2H+(aq)+2Cl−(aq)
The rate equation for this reaction is,
Rate =k[Cl2][H2S]
Which of these mechanisms is/are consistent with this rate equation ?
(I) Cl2+H2S→H++Cl−+Cl++HS− (slow)
Cl++HS−→H++Cl−+S (fast)
(II) H2S⇔H++HS− (fast equilibrium)
Cl++HS−→2Cl−+H++S (slow)
View SolutionAssertion : CH4 does not react Cl2 in dark.
Reason: Chlorination of CH4 takes place in sunlight.
View SolutionConsider the following sequence of reactions CH_(2)=CH_(2)overset(PhCO_(3)H)underset(CH_(2)Cl_(2))(rarr)Aoverset(dil.H_(2)SO_(4))(rarr)B The product (B) is
View SolutionComplete the reaction : CH3COOHCl2/P−−−→?
View SolutionComplete the following reactions .
Sb+Cl2→
View SolutionConsider the reaction,
Cl2(aq)+H2S(aq)→S(s)+2H+(aq)+2Cl−(aq)
The rate equation for this reaction is,
Rate =k[Cl2][H2S]
Which of these mechanisms is/are consistent with this rate equation ?
(I) Cl2+H2S→H++Cl−+Cl++HS− (slow)
Cl++HS−→H++Cl−+S (fast)
(II) H2S⇔H++HS− (fast equilibrium)
Cl++HS−→2Cl−+H++S (slow)
View SolutionThe reaction :
CH3COOAg+Br2CCl4−−→CH3Br+CO2+AgBr
is known as ….
View SolutionWhich of the following reactions would be faster and why? (i) CH_(4)+Cl_(2)^(-) to CH_(3)Cl+HCl (ii) CD_(4)+Cl_(2) to CD_(3)Cl+DCl
View SolutionStatements: Rate of reaction CD_(4)+Cl_(2) to Slow, CH_(4) + Cl_(4) to Intense Reason: Due to isotopic effect
View SolutionThe rate of the reaction 2NO+Cl_(2)rarr 2NOCl is given by the rate equation Rate = k[NO]^(2)[Cl_(2)] The value of the rate constant can be increased by
View SolutionConsider the reaction, Cl_(2)(aq) + H_(2)S(aq) rarr S(s) + 2H^(+) (aq) + 2Cl^(-)(aq) The rate equation for this reaction is, Rate =k [Cl_(2)][H_(2)S] Which of these mechanisms is // are consistent with this rate equation ? (I) Cl_(2) + H_(2)S rarr H^(+) + Cl^(-) + Cl^(+) + HS^(-) (slow) Cl^(+) + HS^(-) rarr H^(+) + Cl^(-) + S (fast) (II) H_(2)S hArr H^(+) +HS^(-) (fast equilibrium) Cl^(+) + HS^(-) rarr 2 Cl^(-) + H^(+) + S (slow)
View SolutionComplete the following chemical equations. CH_(2)=CH_(2)+Br_(2)overset(C Cl_(4))rarr
View Solution
