STATEMENT-1 : H2SO4 can not act as reducing agent.
STATEMENT-2 : Sulphur can not increase its oxidation number beyond +6.
If both Assertion & Reason are True & the Reason is a correct explanation of the Assertion.
If both Assertion & Reason are True but Reason is not a correct explanation of the Assertion.
If Assertion is True but the Reason is False.
If both Assertion & Reason are False.
The correct Answer is:A
Step by step video solution for STATEMENT-1 : H_(2)SO_(4) can not act as reducing agent. STATEMENT-2 : Sulphur can not increase its oxidation number beyond +6. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Oxidation number of Xe in XeF−5 is :
View SolutionWhen ferrous oxalate is titrated against K2Cr2O7, milli equivalents of Fe2+,C2O2−4andCr2O7 in the redox reaction are X,Y and Z respectively , then :-
View SolutionWhich of the following compounds has ionic bond. π−bond, co-ordinate bond and covalent bond:-
View SolutionSTATEMENT-1 : MnO2 can act as an oxidizing agent as well as reducing agent.
STATEMENT-2 : Oxidation state of MnO2 lies between highest and lowest oxidation state.
View SolutionSTATEMENT-1 : H2SO4 can not act as reducing agent.
STATEMENT-2 : Sulphur can not increase its oxidation number beyond +6.
View SolutionSTATEMENT - 1 :
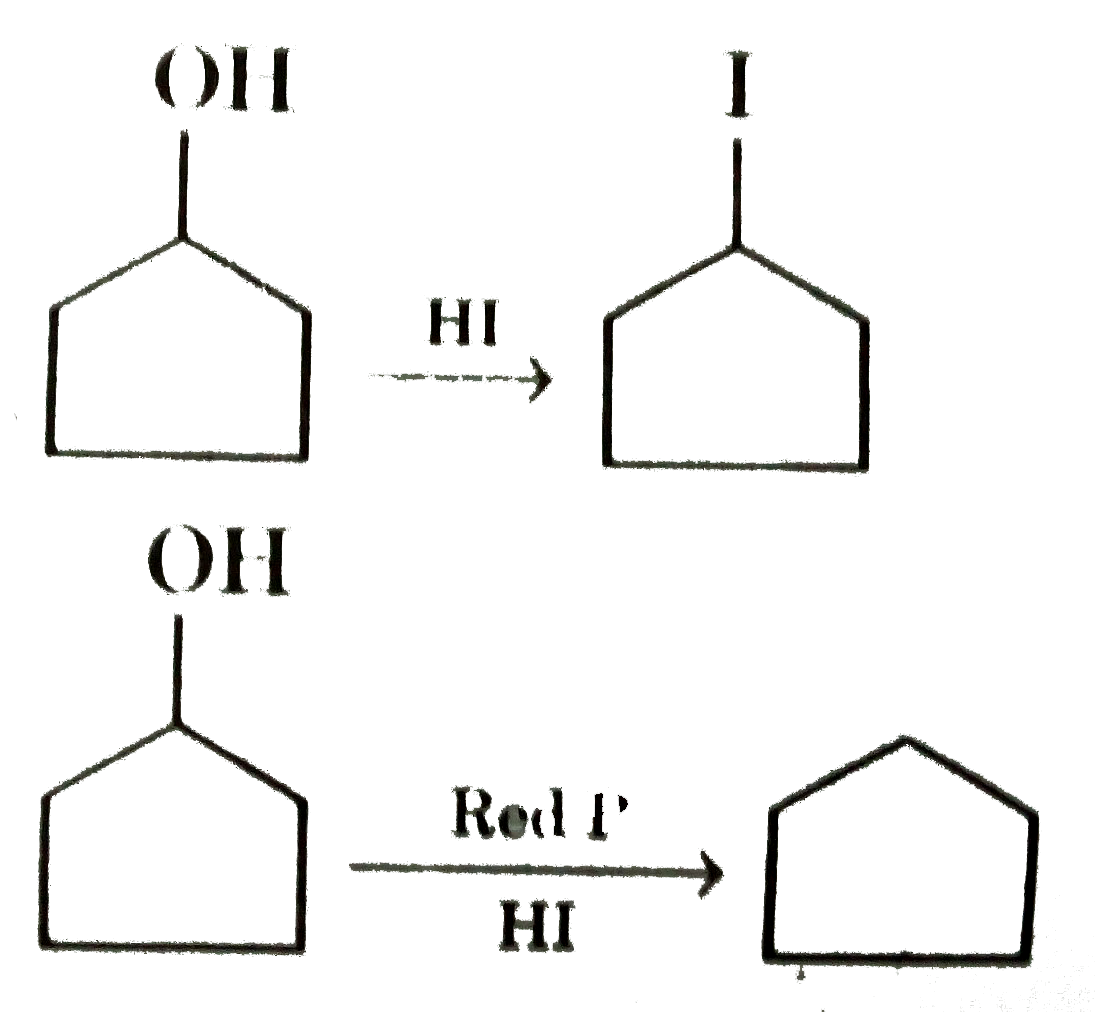 STATEMENT - 2 : Hi can act as reducing agent and red P acts as catalyst for this reaction.
STATEMENT - 2 : Hi can act as reducing agent and red P acts as catalyst for this reaction.
View SolutionH_(2)S acts only as reducing agent while SO_(2) can act both as a reducing agent and as an oxidising agent. How will you account for it ?
View SolutionStatement 1 : H2SO4 cannot act as reducing agent.
Statement 2 : Sulphur cannot increase its oxidation number beyond + 6.
View SolutionSTATEMENT-1 H2O2→H2O+12O2 . This is an example of disproportionation reaction.
STATEMENT-2 H2O2 can act as a oxidising as well as reducing agent .
View SolutionH2S केवल अपचायक की तरह कार्य करती है जबकि SO2 ऑक्सीकारक व अपचायक दोनों की तरह कार्य करती है। क्यों ?
View SolutionStatement-1: [V(CO)6] cannot act as oxidising agent.
Statement-2: It can be reduced by reducing agent.
View SolutionStatement 1 : H2SO4 cannot act as reducing agent.
Statement 2 : Sulphur cannot increase its oxidation number beyond + 6.
View Solutionप्रक्कथन : H2SO4 अपचायक की तरह व्यवहार नहीं कर सकता।
कारण : सल्फर की ऑक्सीकरण संख्या +6 से आगे नहीं बढ़ सकती।
View SolutionThe sulphur oxide SO_(2) , can acts as
View SolutionSTATEMENT-1 : MnO_(2) can act as an oxidizing agent as well as reducing agent. STATEMENT-2 : Oxidation state of MnO_(2) lies between highest and lowest oxidation state.
View SolutionSTATEMENT-1 : H2SO4 can not act as reducing agent.
STATEMENT-2 : Sulphur can not increase its oxidation number beyond +6.
View Solution

