The direct conversion of A to B is difficult hence it is carried out by the following shown path C↑A→D↓B
Given,
ΔS(A→C)=50eu
ΔS(C→D)=30eu
ΔS(B→D)=20eu
where eu is the entropy unit, then ΔS(A→B) is
+100 e.u.
+60 e.u.
−100 e.u.
−60 e.u.
The correct Answer is:B
ΔS(A→B)=ΔS(A→C)+ΔS(C→D)−ΔS(B→D)
=50+30−20=60 e.u.
Topper's Solved these Questions
- THERMODYNAMICS BOOK - ALLENCHAPTER - THERMODYNAMICS EXERCISE - EXERCISE -2
100 Videos
- THERMODYNAMICS BOOK - ALLENCHAPTER - THERMODYNAMICS EXERCISE - EXERCISE -3
108 Videos
- THERMODYNAMICS BOOK - ALLENCHAPTER - THERMODYNAMICS EXERCISE - EXERCISE -1A
41 Videos
- TEST PAPERSBOOK - ALLENCHAPTER - TEST PAPERSEXERCISE - MATHEMATICS
2 Videos
- Thermodynamics And Thermo ChemistryBOOK - ALLENCHAPTER - Thermodynamics And Thermo ChemistryEXERCISE - All Questions
40 Videos
Similar Questions
The first law of thermodynamics is concerned with the conservation of
View SolutionAn exothermic reaction is one in which the reacting substances :
View SolutionThe magnitude of enthalpy change for neutralization of the reaction ,
MgO(s)+2HCl(aq)→MgCl2(aq)+H2O(l) (Given H+(aq)+OH−(aq)→H2O(l),−57 kJ/mol) will be :
View SolutionWhich of the following oxide is basic?
View SolutionGiven the following entropy values ( in JK−1mol−1) at 298 K and 1 atm: H2(g):130.6,Cl2(g):223.0,HCl(g):186.7. The entropy change (in JK−1mol−1) for the reaction
H2(g)+Cl2(g)→2HCl(g) , is
(a)+540.3
(b)+727.0
(c)−166.9
(d)+19.8
View SolutionThe direct conversion of A to B is difficult, hence it is carried out as A rarr C rarrD rarr B Given, DeltaS_((A^rarrC)) = 50 eU,DeltaS_((C^rarrD))=30eU,DeltaS_((BrarrD))=20 eU , where eU is entropy unit. Thus the change in entropy in (A rarrB) is:
View Solution'A'+NaOH→'B'(gas)+'C'Zn−−→'B'
'B'(gas)[O]−−→'D'(gas)Mg−−→Δ'E'(White)H2O−−→'B'(gas)
The gas D and B are respectively?
View SolutionThe conversion A to B is carried out by the following path : C→D↑ ↓A B Given : ΔS(A→C)=50 e.u. ,
ΔS(C→D)=30 e.u., ΔS(B→D)=20 e.u.
where w.u. is entropy unit then ΔS(A→B) is
View SolutionThe direct conversion of A to B is difficult. Hence, it is carried out by the following shown path : DeltaS_((A rarr C)) =50 e.u., DeltaS _((C rarr D)) = 30 e.u. DeltaS_((BrarrD)) = 20 e.u. where e.u. is entropy unit, then DeltaS _((A rarr B )) is
View SolutionThe direct conversion of A or B is difficult, hence it is carried out by the following shown path
Given, ΔSA→C=50eu, ΔSC→D=30eu,ΔSB→D=20eu
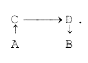
Where eu is entropy unit, then ΔSA→B is .
View SolutionThe conversion A to B is carried out by the following path : C→D↑ ↓A B Given : ΔS(A→C)=50 e.u. ,
ΔS(C→D)=30 e.u., ΔS(B→D)=20 e.u.
where e.u. is entropy unit then ΔS(A→B) is
View SolutionA का B में सीधा परिवर्तन बहुत कठिन है। अतः इस परिवर्तन को निम्न प्रकार किया जाता है
दिया है
ΔSA→C=50eu,ΔSC→D=30eu,ΔSB→D=20eu
जहाँ, eu एन्ट्रॉपी की इकाई है। यहाँ ΔSA→B होगा
View Solution(C(A)H3COOHPCl5−−→[B]),CH3COOHNaOH−−−→[C]),[B]+[C]→[D]):}
यौगिक [B], [C] तथा [D] है |
View SolutionThe reaction between A to B is not feasible but on changing entropy through a series of steps : A to C to D to B DeltaS (A to C) = 50 eu DeltaS (C to D) = 30 eu DeltaS(B to D) = 20 eu . The entropy change for A to B would be :
View SolutionThe direct conversion of A to B is difficult, hence it is carried out by the following shown path A→C→D→B. Given ΔSA→C= 50 eu, ΔSC→D= 30 eu, ΔSB→D= 20eu
View SolutionA का B में सीधा परिवर्तन बहुत कठिन है। अतः इस परिवर्तन को निम्न प्रकार किया जाता है
 दिया है,
दिया है,
ΔS(A→C)=50eu
ΔS(C→D)=30eu
ΔS(B→D)=20eu
जहाँ, eu एन्ट्रॉपी की इकाई है। यहाँ ΔS(A→B) होगा
View Solution

