Which of the following has the least negative heat of combustion?
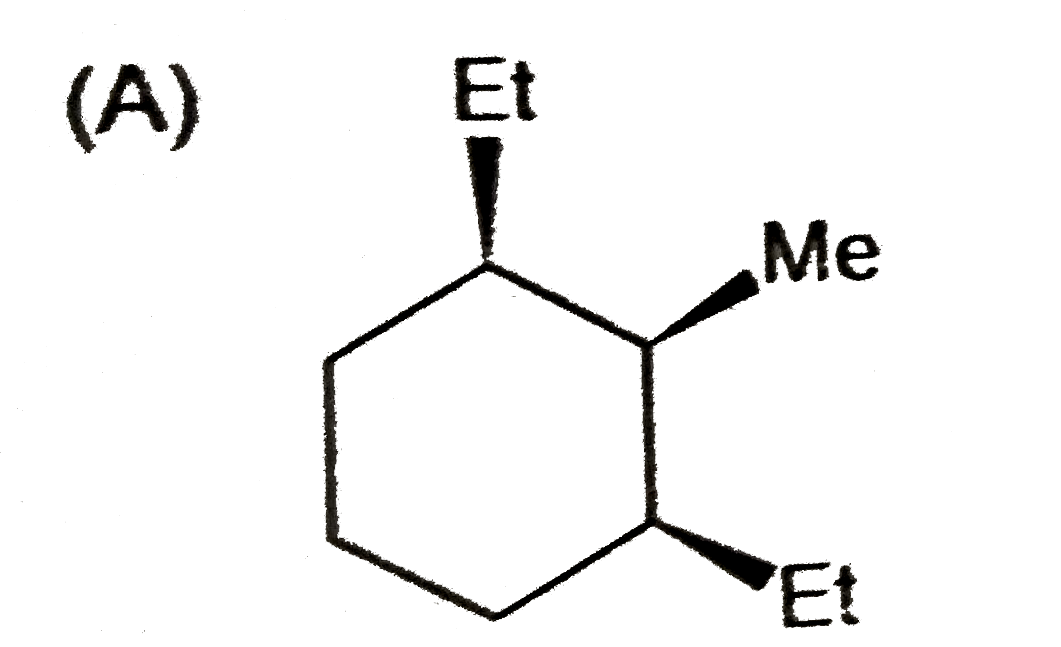
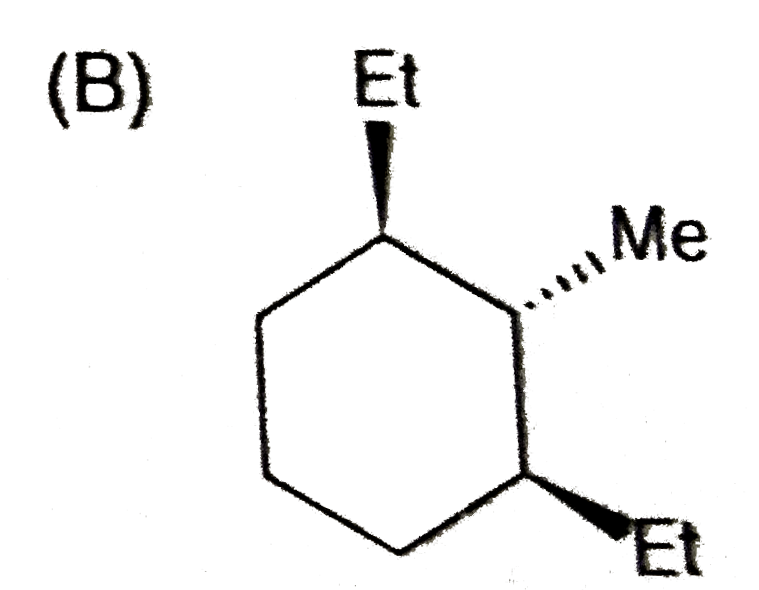
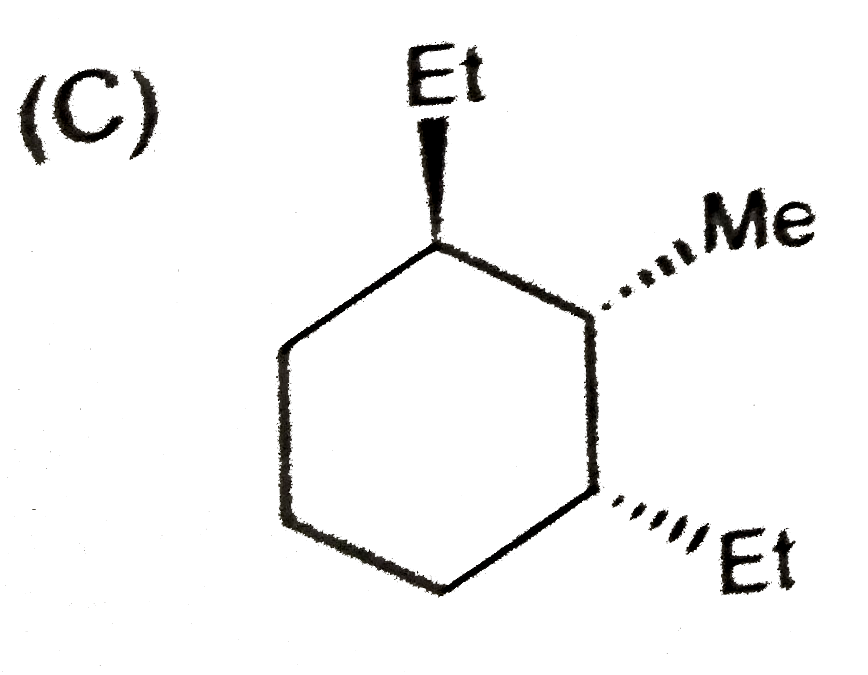
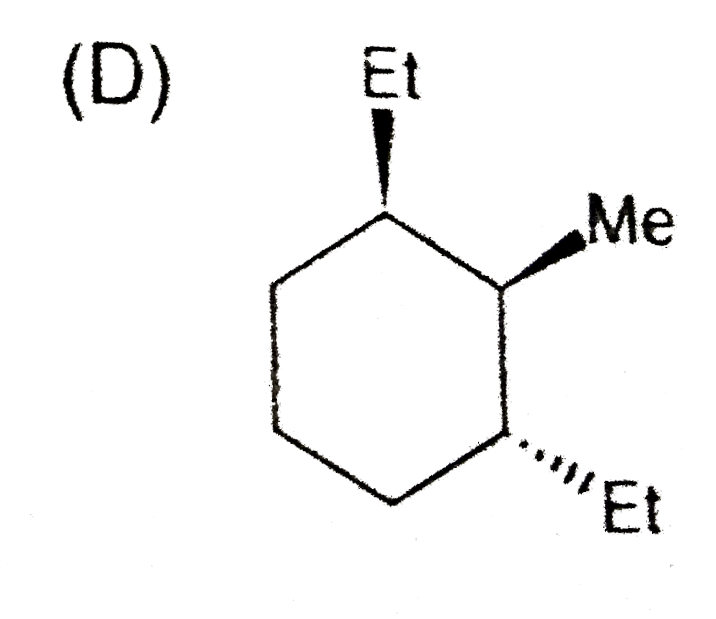
The correct Answer is:B
To determine which of the given compounds has the least negative heat of combustion, we need to analyze the stability of each compound based on the arrangement of substituents. The stability of a compound is inversely related to its heat of combustion; the more stable a compound is, the less negative its heat of combustion will be.
1. Understand Heat of Combustion:
- Heat of combustion refers to the energy released when a substance undergoes complete combustion. A more negative value indicates a greater release of energy and thus less stability.
- Hint: Remember that a more negative heat of combustion means the compound is less stable.
2. Identify the Compounds:
- We need to look at the given options and identify the structural arrangement of substituents on each compound.
3. Analyze Substituent Arrangement:
- For each compound, observe how the substituents are arranged (axial or equatorial) and whether they are on the same plane or not.
- Hint: Compounds with substituents on the same plane tend to have higher steric repulsion, leading to lower stability.
4. Evaluate Stability:
- Option A: All substituents are on the same side (above the plane), leading to high steric repulsion and low stability.
- Option B: Substituents are arranged such that they minimize steric interactions (some above and some below), indicating higher stability.
- Option C: Two substituents are on the same plane, leading to some steric repulsion, thus lower stability than B.
- Option D: Similar to C, with two substituents on the same plane, leading to lower stability than B.
- Hint: The arrangement that minimizes steric hindrance will generally be the most stable.
5. Determine the Compound with Least Negative Heat of Combustion:
- After evaluating the stability of all options, it is clear that Option B has the highest stability due to its optimal arrangement of substituents.
- Therefore, Option B will have the least negative heat of combustion compared to the others.
Conclusion:
The compound with the least negative heat of combustion is Option B.
- Heat of combustion refers to the energy released when a substance undergoes complete combustion. A more negative value indicates a greater release of energy and thus less stability.
- Hint: Remember that a more negative heat of combustion means the compound is less stable.
- We need to look at the given options and identify the structural arrangement of substituents on each compound.
- For each compound, observe how the substituents are arranged (axial or equatorial) and whether they are on the same plane or not.
- Hint: Compounds with substituents on the same plane tend to have higher steric repulsion, leading to lower stability.
- Option A: All substituents are on the same side (above the plane), leading to high steric repulsion and low stability.
- Option B: Substituents are arranged such that they minimize steric interactions (some above and some below), indicating higher stability.
- Option C: Two substituents are on the same plane, leading to some steric repulsion, thus lower stability than B.
- Option D: Similar to C, with two substituents on the same plane, leading to lower stability than B.
- Hint: The arrangement that minimizes steric hindrance will generally be the most stable.
- After evaluating the stability of all options, it is clear that Option B has the highest stability due to its optimal arrangement of substituents.
- Therefore, Option B will have the least negative heat of combustion compared to the others.
The compound with the least negative heat of combustion is Option B.
Similar Questions
Knowledge Check
Which of the following has the most negative heat of hydrogenation ?
A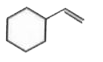 B
B C
C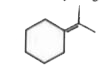 D
D
Which one of the following has the smallest heat of combustion ?
A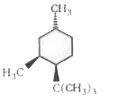 B
B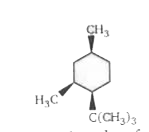 C
C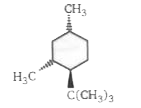 D
D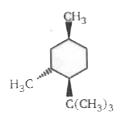
Which of the following has the lowest heat of combustion?
A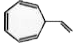 B
B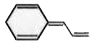 C
C D
D
Which of the following has the least polarity in the bond?
View SolutionWhich of the following has the highest negative electron gain entahlpy ?
View SolutionWhich of the following has the least ionization potential ?
View SolutionWhich of the following cycloalkanes has lowest heat of combustion per −CH2− group per mole?
View SolutionWhich one of the following has lowest heat of combustion?
View Solution

