For the ion [ICl2]−, the only correct combination is
A
(III)(i)(S)
B
(II)(iii)(Q)
C
(IV)(iv)(R)
D
(IV)(i)(R)
Video Solution
Text Solution
The correct Answer is:A
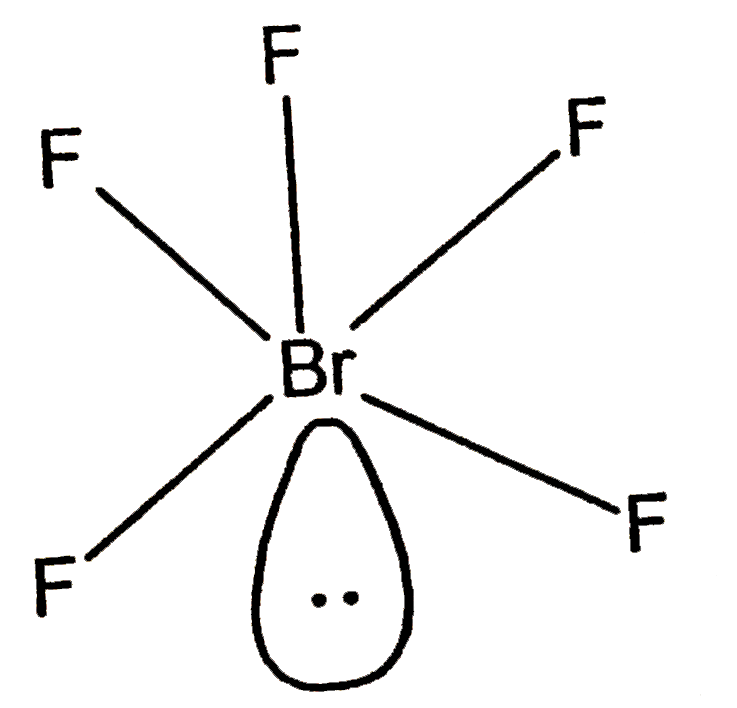
Hybridisation −sp2d2
Shape - square pyramidal
Lone pair in central atom =1
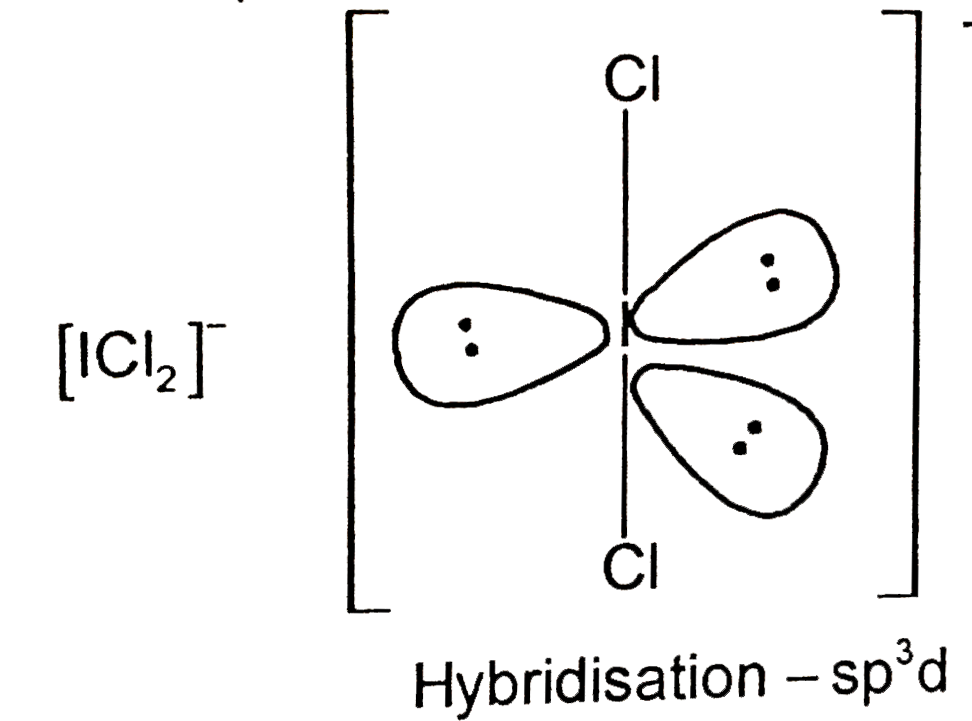
Shape -Linear
Lone pair in the central atom =3
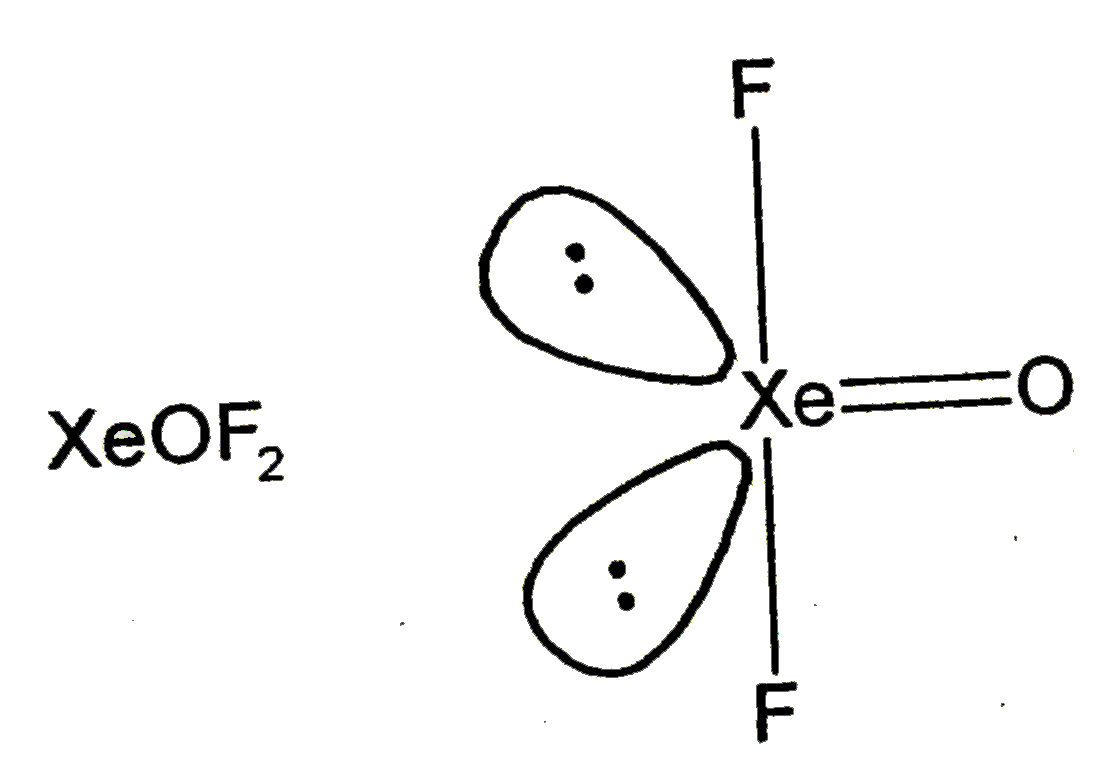
Shape-T shaped
Lone pair in the central atom =2
|
Similar Questions
Knowledge Check
Select the correct combination:
AH3C−O∣∣C−HϴOH−−−−−→ΔH3C−CH=CH−O∣∣C−HBPh−O∣∣C−HKOH−−→Ph−O∣∣C−ϴOK⊕+Ph−CH2−OHCPh−O∣∣C−Al(OEt)3−−−−−→Ph−O∣∣C−O−CH2−PhDH−O∣∣C−HNaOH−−−→H−O∣∣C−OH+CH3OH
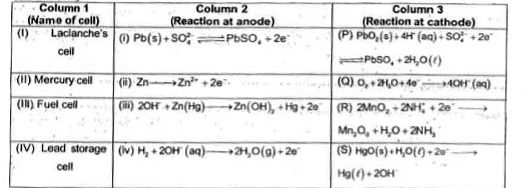
The correct combination is
View SolutionThe correct combination is :
View Solution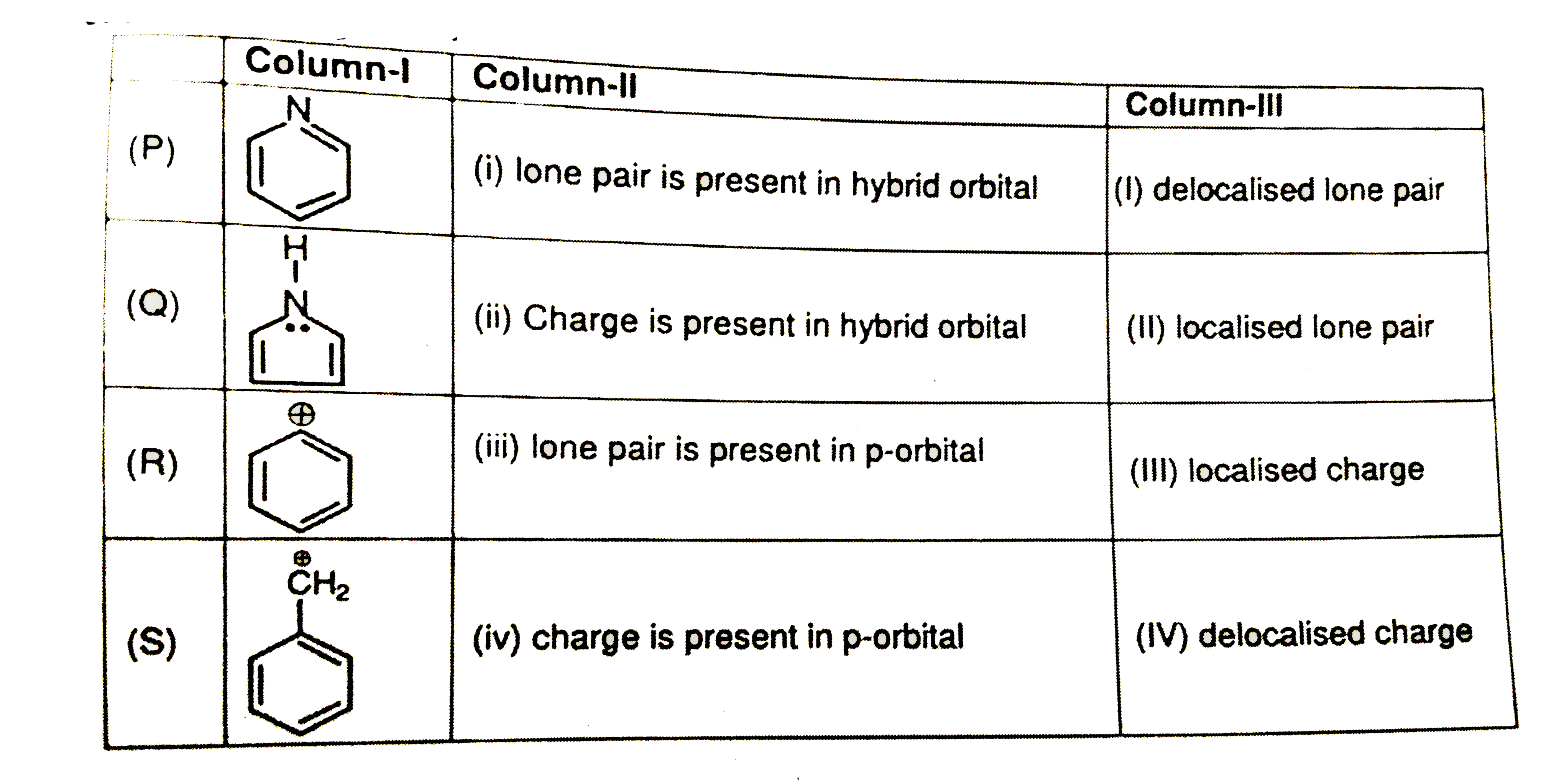
The only correct combination for pyrrole is
View SolutionChoose the correct combination
View SolutionChoose the correct combination
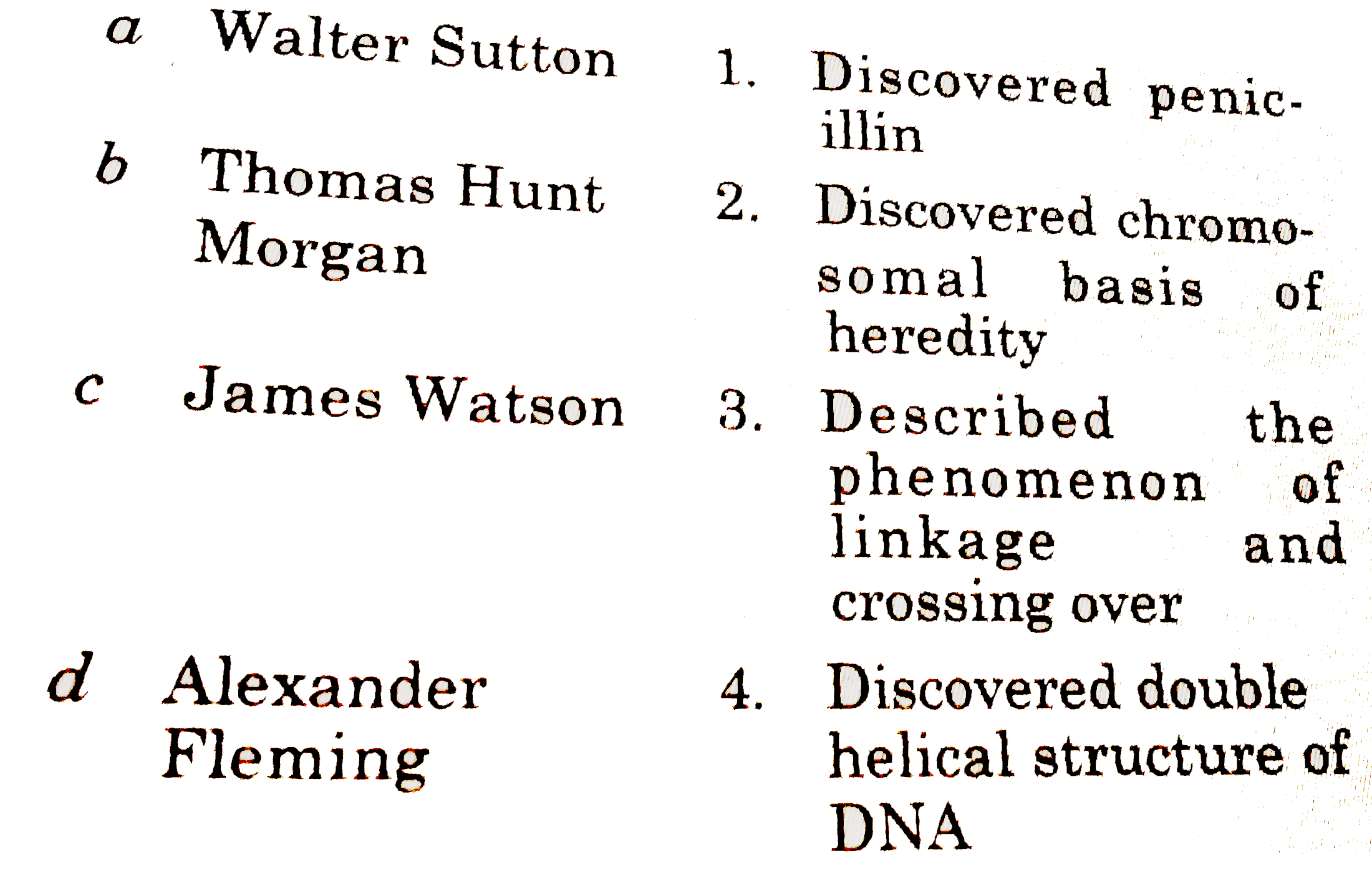
View Solution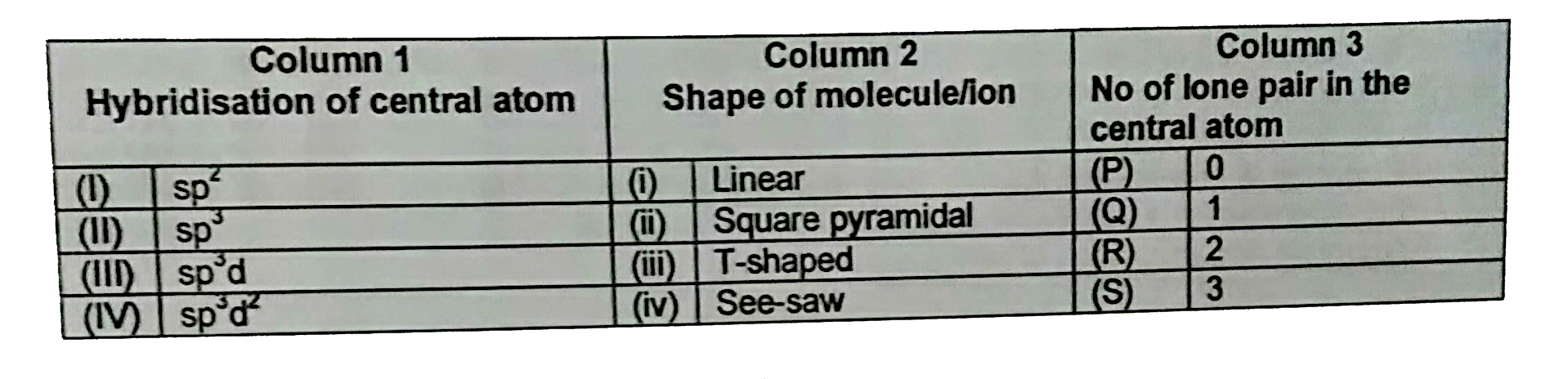
For the molecule XeOF2, the only correct combination is
View SolutionChoose the correct combination.
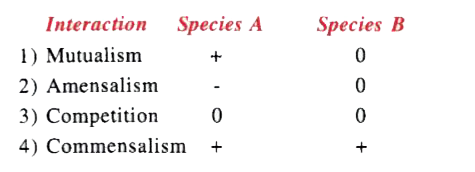
View Solution