A compound containing carbon, hydrogen and oxygen gave the following analytical data C= 40.0%, H= 6.67%. Calculate the molecular formula of the compound, If its molecular mass is 180.Solution in Punjabi
We are given that
(i) Percentae of carbon = 40.0%
Percentage of hydrogen = 6.67%
(ii) Molecular mass of compound = 180
We are to find out the following
(a) Percentage of oxygen = ?
(b) Empirical formula mass= ?
( c) Value of n= ?
(d) Molecular formula of compound= ?
Calculations
(i) Calculation of percentage of oxygen =100−(% of C+% ofH)
=100−(40.0+6.67)=53.5%
(ii) Calculate of empirical formula mass of the compound
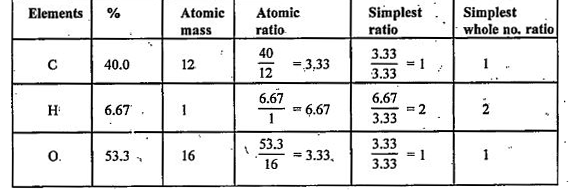
Empirical formula of the compound =C1H2O1=CH2O
∴ Empirical formula mass of the compound =12+2+16=30
(iii) Calculation of value of n=Molecular formula massEmpirical formula mass=18030=6
(iv) Molecular formula of compound =n× empirical formula =6×(C1H2O1)1=C6H12O6. Thus, molecular formula of the compound is C6H12O6.
Topper's Solved these Questions
- SOME BASIC CONCEPTS OF CHEMISTRYBook:OMEGA PUBLICATIONChapter:SOME BASIC CONCEPTS OF CHEMISTRYExercise:Multiple Choice Questions (MCQs)
Explore 29 Videos
- SAMPLE QUESTION PAPER -v (PUNJAB)Book:OMEGA PUBLICATIONChapter:SAMPLE QUESTION PAPER -v (PUNJAB)Exercise:QUESTION
Explore 45 Videos
- STATES OF MATTER Book:OMEGA PUBLICATIONChapter:STATES OF MATTER Exercise:Multiple Choice Questions (MCQs)
Explore 29 Videos
Similar Questions
How many significant figures are there in each of the following?
6.022×1023
View SolutionHard water contains :
View SolutionGas equation PV = nRT is obeyed by
View Solutionकिसी रासायनिक यौगिक में कार्बन 40% तथा हाइड्रोजन 6.67%है. यदि यौगिक का अणुभार 30 हो तो उसके मुलानुपाती तथा आणविक सूत्र ज्ञात कीजिए.
View SolutionA compound of carbon, hydrogen and oxygen contains 40% of carbon and 6.67% of hydrogen. Vapour density of the compound is 2.813 times of the vapour density of oxygen. Determine the empirical formula and molecular formula of the compound.
View SolutionCalculate the empirical and molecular formula of the compound containing 80% Carbon, 20% Hydrogen. If the molecular mass of the compound is 30 then determine the molecular formula.
View SolutionA carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula.
View SolutionAn organic compound containing carbon, hydrogen and oxygen gave the following percentage composition :
C = 40.68%, H = 5.08%
The vapour density of the compound is 59. Calculate the molecular formula of the compound.
View SolutionC = 40%, H = 6.67% in a compound of carbon, hydrogen and oxygen. Determine the coarse signal and molecular signal of the compound
View SolutionA compound contains carbon hydrogen and nitrogen in the ratio 18:2:7 Calculate its empirical formula. If molecular mass of the compound is 108 What is the molecular formula ?
View SolutionA compound contains carbon hydrogen and nitrogen in the ratio 18:2:7 Calculate its empirical formula. If molecular mass of the compound is 108 What is the molecular formula ?
View SolutionA compound contains 80% of carbon and 20% hydrogen. If the molecular mass is 30 calculate empirical formula and molecular formula.
View SolutionAn organic compound on analysis gave the following composition. Carbon=40%, Hydrogen=6.66% and oxygen= 53.34%. Calculate its molecular formula if its molecular mass is 90.
View SolutionAn organic compound on analysis gave the following composition: carbon = 40%, hydrogen = 6.66% and oxygen = 53.34%. Calculate its molecular formula if its molecular mass is 90.
View SolutionA compound is composed of 2.2% hydrogen, 26.6% carbon and 71.2% oxygen. Calculate the empirical formula of the compound. If its molecular mass is 90, find its molecular formula.
View SolutionA carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula.
View Solution
