Alcohols are easily protonated in comparison to phenols.Solution in Punjabi
In alcohols the lone pair of electron on oxygen is localised and hence is easily available for protonation.
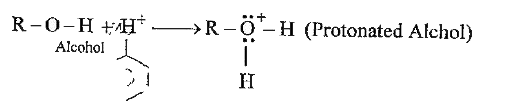
On the other hand, the lone pair of electrons on oxygen atom in phenols is involved in resonance and hence is delocalised and thus not easily available for protonation. Therefore alcohols are easily protonated in comparison to phenol.
Topper's Solved these Questions
- ALCOHOL, PHENOL AND ETHERBook:ACCURATE PUBLICATIONChapter:ALCOHOL, PHENOL AND ETHERExercise:ETHERS
Explore 16 Videos
- ALCOHOL, PHENOL AND ETHERBook:ACCURATE PUBLICATIONChapter:ALCOHOL, PHENOL AND ETHERExercise:TRUE AND FALSE (1 MARK)
Explore 15 Videos
- MODEL TEST PAPER-6Book:ACCURATE PUBLICATIONChapter: MODEL TEST PAPER-6Exercise: SECTION-D (LONG ANSWER QUESTIONS) (TYPE II)
Explore 16 Videos
- ALDEHYDES ,KETONES AND CARBOOXALIC ACIDSBook:ACCURATE PUBLICATIONChapter:ALDEHYDES ,KETONES AND CARBOOXALIC ACIDSExercise:DISTINGUISH TEST
Explore 5 Videos
Similar Questions
What are ethers ?
View SolutionComplete and balance equation : NaOH + HCl →
View SolutionDiscuss oxidation of primary,secondary and tertiary alcohols.
View SolutionDiscuss iodoform test. How will you distinguish between propane-1-ol and Propane-2-ol with this test ?
View SolutionAssertion: Alcohols are easily protonated thena phenols. Reason: Alcohols undergo intermolecular hydrogen bonding due to the presence of highly electornegative oxygen.
View SolutionGive possible explanation for the following. (a) Ortho-nitrophenol is more acidic than ortho-methoxyphenol. (b) Alcohols are easily protonated in comparision to phenols. (c) The relative ease of dehydration of alcohols is : tertiary gt secondary gt primary.
View SolutionAlcohols can be easily distinguished from phenol because
View SolutionPhenol easily dissolves in _________.
View SolutionPhenols and alcohols are respectively ________
View SolutionUnlike phenols, alcohols are easily protonated. Or Alcohols are easily protonated in comparison to phenols. Or Why do phenols not give protonation reactions readily?
View SolutionHaloalkanes can easily be prepared from alcohols while aryl halides cannot be prepared from phenol. Explain.
View Solutionफीनॉल का प्रोटोनीकरण कठिन है जबकि एथेनॉल का प्रोटोनीकरण आसानी से किया जाता है कारण दीजिए-
View Solution−COOH समूह ऐल्कोहॉल के −OH समूह की तुलना में आसानी से प्रोटॉन क्यों देता है?
View SolutionUnlike phenols, alcohols are easily protonated.
View SolutionChemical Properties Of Alcohol And Phenol
View Solution(i) Phenol has higher boiling point than toluene. Why?
(ii) Why are alcohols easily protonated but phenols are not protonated?
View Solution
