How many unpaired electrons are present in Fe+3 and Zn+2.Solution in Punjabi
Electronic configuration of Fe 26 (3d6 4s2)
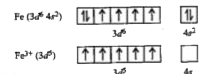
In Fe3+, there are unpaired
The electronic configuration of Zn. (30) = 3d10 4s2.

In Zn2+, there are no unpaired electrons.
टॉपर्स ने हल किए ये सवाल
- SOLVED MODEL TEST PAPER-1Book:ACCURATE PUBLICATIONChapter:SOLVED MODEL TEST PAPER-1Exercise:SECTION C
Explore 11 Videos
- SOLVED SAMPLE PAPER, MARCH - 2021 Book:ACCURATE PUBLICATIONChapter:SOLVED SAMPLE PAPER, MARCH - 2021 Exercise:SECTION -D
Explore 16 Videos
- SOLVED MODEL TEST PAPER-2Book:ACCURATE PUBLICATIONChapter:SOLVED MODEL TEST PAPER-2Exercise:SECTION D
Explore 11 Videos
समान प्रश्न
The molar conductivities at infinite dilution for sodium acetate, _hydrochloric acid and sodium cholride are 92.5,426.9 and 120.4 Scm2mol−1 respectively at 298 K. Calculate the molar conductivity of acetic acid at infinite dilution.
View SolutionExplain, why transition metal ions usually show paramagnetic behaviour ?
View SolutionExplain Swarts reaction.
View SolutionHow many unpaired electrons are present in NO molecule ?
View SolutionHow many unpaired of electron are present in O−2
View SolutionHow many unpaired electrons are present in a ground state?
View SolutionHow many unpaired electrons ae in a Fe^(2+) ion in the ground state?
View SolutionHow many unpaired electrons are in a Fe^(2+) ion in the ground state?
View SolutionHow many unpaired electrons are present in chromium (Cr)
View SolutionHow many unpaired electrons are present in oxygen molecule ?
View SolutionHow many unpaired electrons are present in N+2 ?
View SolutionHow many unpaired electrons are present in Co3+,Fe2+? Calculate their magnetic moment.
View SolutionHow many unpaired electrons are present in central atom of [Fe("ox")_(3)]^(3-) ?
View SolutionHow many unpaired electrons are present in each of the following ?
Fe3+,Co2+,Zn2+
View SolutionFe+2 तथा Fe+3 में अयुग्मित इलेक्ट्रॉन की संख्या है।
View SolutionHow many unpaired electrons are present in N+2
View Solution
