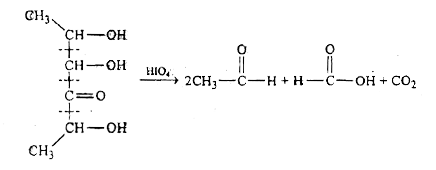An organic compound A (Molecular formula C6H12O4) on treatment with Na metal liberates H2 gas and on treatment with HIO4 gives 2 moles of CH3CHO,HCOOH (1 mole) and CO2 (1 mole). Find out structure of A.
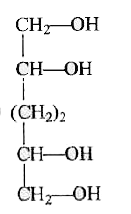
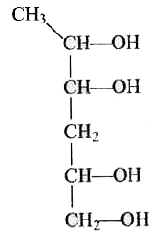
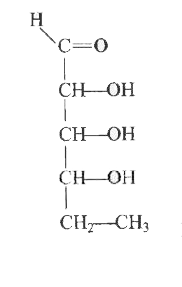
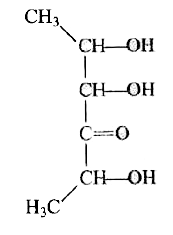
The correct Answer is:D
Similar Questions
2moles of H_(2)O_(2) is treated with equivalent amount of KMnO_(4) in the presence of H_(2)SO_(4) then moles of O_(2) gas liberated is
View SolutionAn organic compound 'X' of molecular formula C6H12 , absorbs one mole of bromine to give C6H12Br2 Onreduction it gives 2-methylpentane, while on oxidation yieldsa mixture of acetic acid and isobutyric acid. Derive the structural formula of X and give its IUPAC name.
View SolutionCalculate the molecular masses of H2,O2,Cl2,CO2,CH4,C2H6,C2H4,NH3,CH3OH.
View SolutionAn organic compound ‘A’ has molecular formula C9H13NO and it can be resolve into enantiomers. Adoes not decolourise bromine water solution. A on refluxing with dilute H2SO4, yields another revolvable compound B(C9H14O3) which give effervescence with NaHCO3. B on treatment with NaBH4 yields C(C9H16O3) on heating with concentrated H2SO4 yields ester D (C6H14O2).
Compound A on reduction with LiAlH4 followed by treatment of H2SO4 yields following compound :
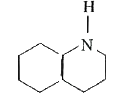
Find out structure of compound 'A''
View SolutionCalculate the molecular masses of H2,O2,Cl2,CO2,CH4,C2H6,C2H4,NH3,CH3OH.
View SolutionOne mole of hydrocarbon (A) reacts with one mole of bromine giving a dibromocompound C_(5)H_(10)Br_(2) . Substance (A) on treatment with cold, dilute alkaline KMnO_(4) solution forms a compound C_(5)H_(12)O_(2) . On ozonolysis, (A) gives equimolar quantities of propanone and ethanal. Deduce the structural formula of (A)
View SolutionMixture of 1 mole of each CH_(4) and C_(2)H_(6) absorb 5 mole of Cl_(2) to form C Cl_(4) and C_(2)Cl_(6) . Then average molecular wt. of the gaseous mixture is
View SolutionA gaseous alkane on complete combustion gives CO_(2) and H_(2)O . If the ratio of moles O_(2) needed for combustion and moles of CO_(2) formed is 5:3 find out the formula of alkane.
View Solution