CO2 gas adsorbs on charcoal following Freundlich adsorption isotherm. For a given amount of charcoal, the mass of CO2 adsorbed becomes 64 times when the pressure of CO2 is doubled.
The value of n in the Freundlich isotherm equation is ________ ×10−2. (Round off to the Nearest Integer)
The correct Answer is:17
To solve the problem, we will use the Freundlich adsorption isotherm equation, which is given by:
xm=kp1n
where:
- x is the mass of the gas adsorbed,
- m is the mass of the adsorbent (charcoal),
- k is a constant,
- p is the pressure of the gas,
- n is a constant that indicates the adsorption intensity.
Step 1: Set up the initial conditions
Let:
- x1 be the mass of CO2 adsorbed at pressure p1.
- x2 be the mass of CO2 adsorbed at pressure p2.
According to the problem, when the pressure is doubled, the mass of CO2 adsorbed becomes 64 times the initial amount:
x2=64x1
Step 2: Express the pressures
Since the pressure is doubled, we have:
p2=2p1
Step 3: Write the Freundlich isotherm equations for both states
Using the Freundlich isotherm for both conditions, we have:
1. For p1:
x1m=kp1n1
2. For p2:
x2m=k(2p1)1n
Step 4: Substitute x2 in terms of x1
Substituting x2=64x1 into the second equation gives:
64x1m=k(2p1)1n
Step 5: Divide the two equations
Dividing the second equation by the first equation:
64x1/mx1/m=k(2p1)1nkp1n1
This simplifies to:
64=(2p1)1np1n1
Step 6: Simplify the right side
This can be simplified as follows:
64=21n
Step 7: Solve for n
Taking logarithms on both sides:
log(64)=1nlog(2)
Since 64=26, we have:
6log(2)=1nlog(2)
Dividing both sides by log(2):
6=1n
Thus, we find:
n=16
Step 8: Convert n to the required format
To express n in the form of xx×10−2:
n=16=0.1666...≈0.17×10−2
Step 9: Round off to the nearest integer
The nearest integer to 0.17×10−2 is 17.
Final Answer
The value of n in the Freundlich isotherm equation is:
17
- x is the mass of the gas adsorbed,
- m is the mass of the adsorbent (charcoal),
- k is a constant,
- p is the pressure of the gas,
- n is a constant that indicates the adsorption intensity.
Let:
- x1 be the mass of CO2 adsorbed at pressure p1.
- x2 be the mass of CO2 adsorbed at pressure p2.
Since the pressure is doubled, we have:
Using the Freundlich isotherm for both conditions, we have:
x1m=kp1n1
x2m=k(2p1)1n
Substituting x2=64x1 into the second equation gives:
Dividing the second equation by the first equation:
This can be simplified as follows:
Taking logarithms on both sides:
To express n in the form of xx×10−2:
The nearest integer to 0.17×10−2 is 17.
The value of n in the Freundlich isotherm equation is:
Topper's Solved these Questions
- JEE MAINS 2021Book:JEE MAINS PREVIOUS YEARChapter:JEE MAINS 2021Exercise:Chemistry Section A
Explore 40 Videos
- JEE MAINS 2021Book:JEE MAINS PREVIOUS YEARChapter:JEE MAINS 2021Exercise:Chemistry Section B
Explore 20 Videos
- JEE MAINS 2021Book:JEE MAINS PREVIOUS YEARChapter:JEE MAINS 2021Exercise:CHEMISTRY (SECTION-A)
Explore 80 Videos
- JEE MAINS 2020Book:JEE MAINS PREVIOUS YEARChapter:JEE MAINS 2020Exercise:CHEMSITRY
Explore 25 Videos
- JEE MAINS 2022Book:JEE MAINS PREVIOUS YEARChapter:JEE MAINS 2022Exercise:CHEMISTRY (SECTION-B)
Explore 10 Videos
Similar Questions
Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot,x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P. xm is proportional to:
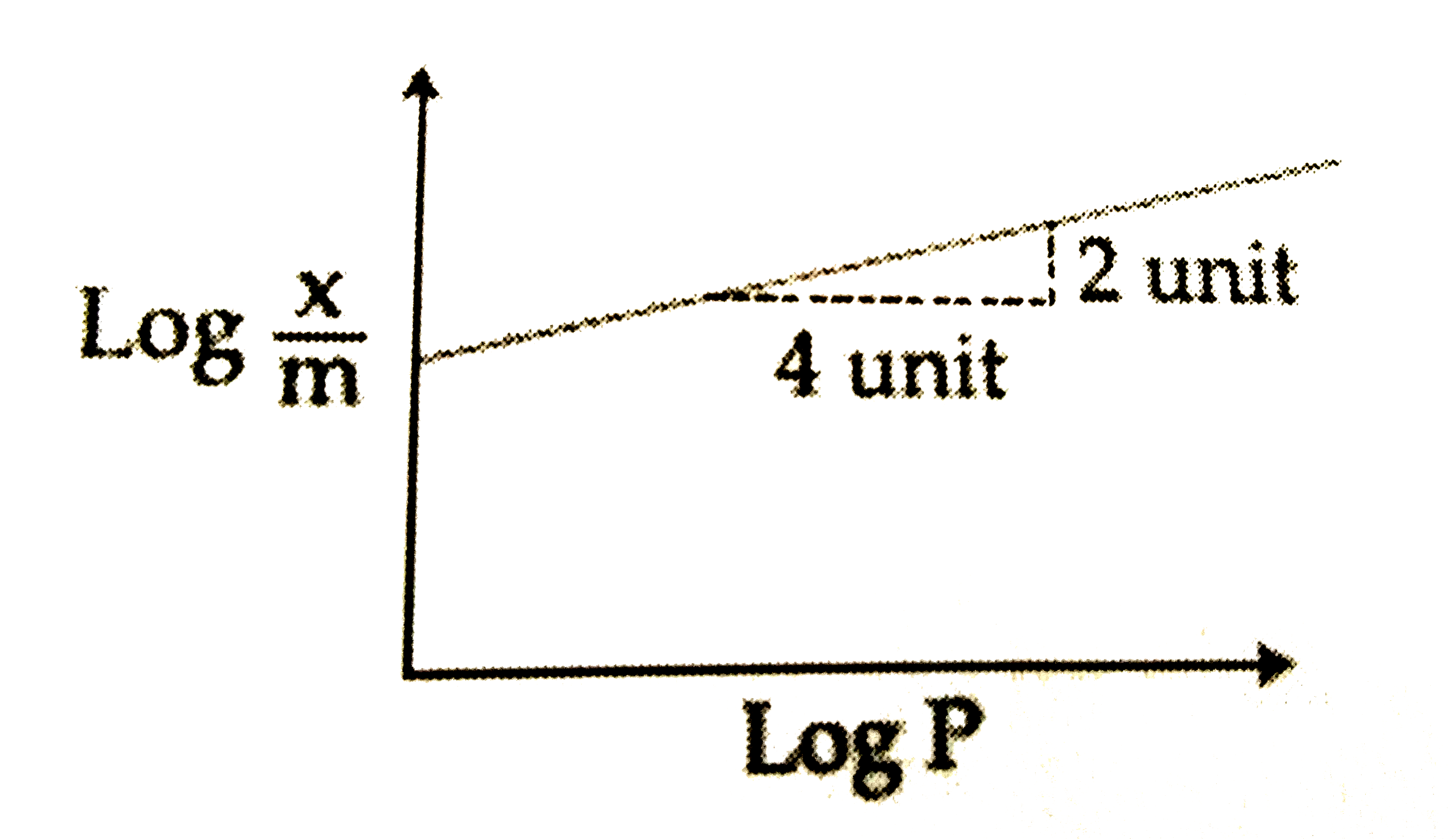
View SolutionAdsorption of gas follows Freundlich adsorption isotherm. If x is the mass of the gas adsorbed on mass m of the adsrobent , the correct plot of xm versus p is :
View Solution
Knowledge Check
Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot, x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p. xm is proportional to :
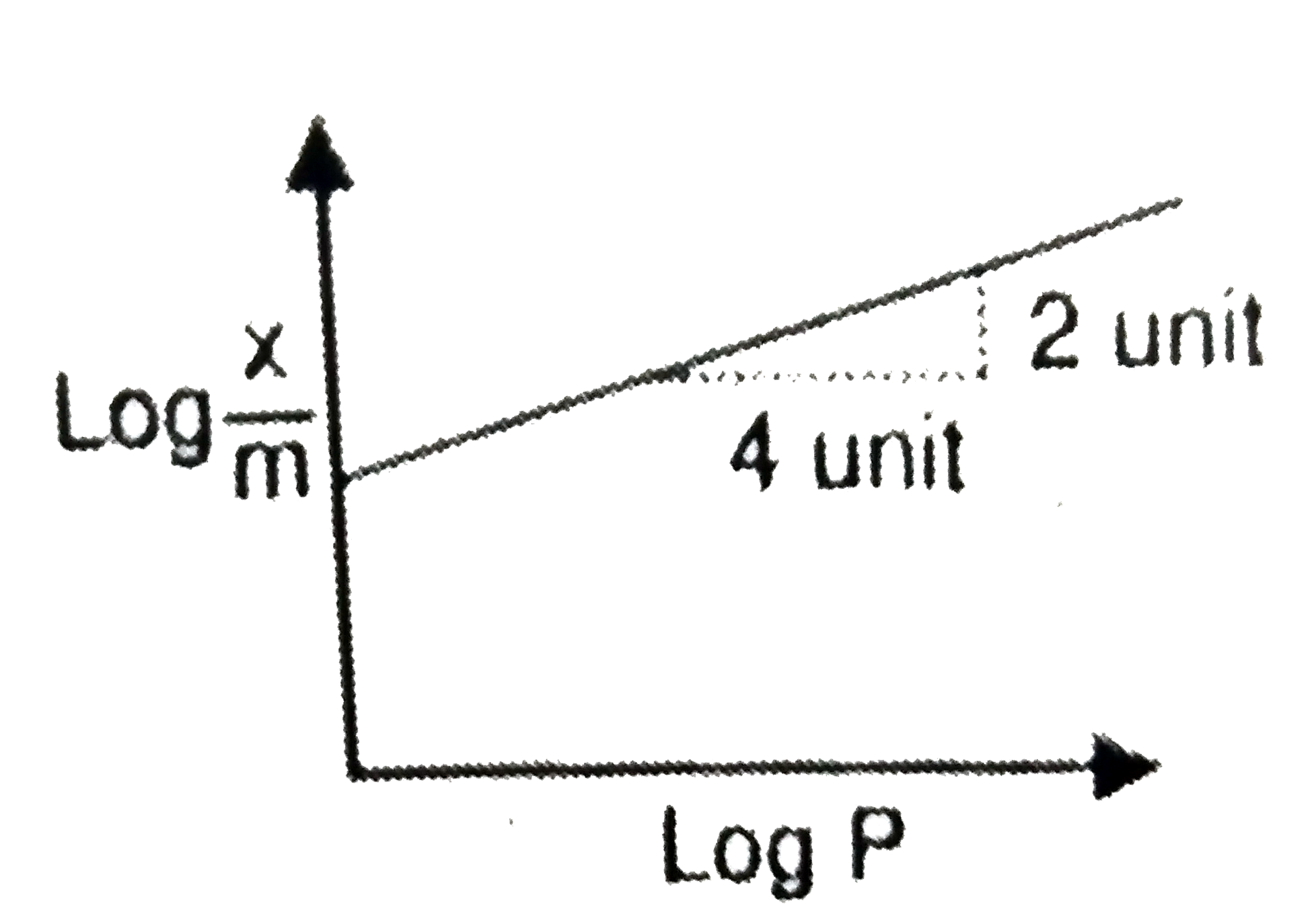 Ap2BpCp1/2Dp1/4
Ap2BpCp1/2Dp1/4Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot,x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P. xm is proportional to:
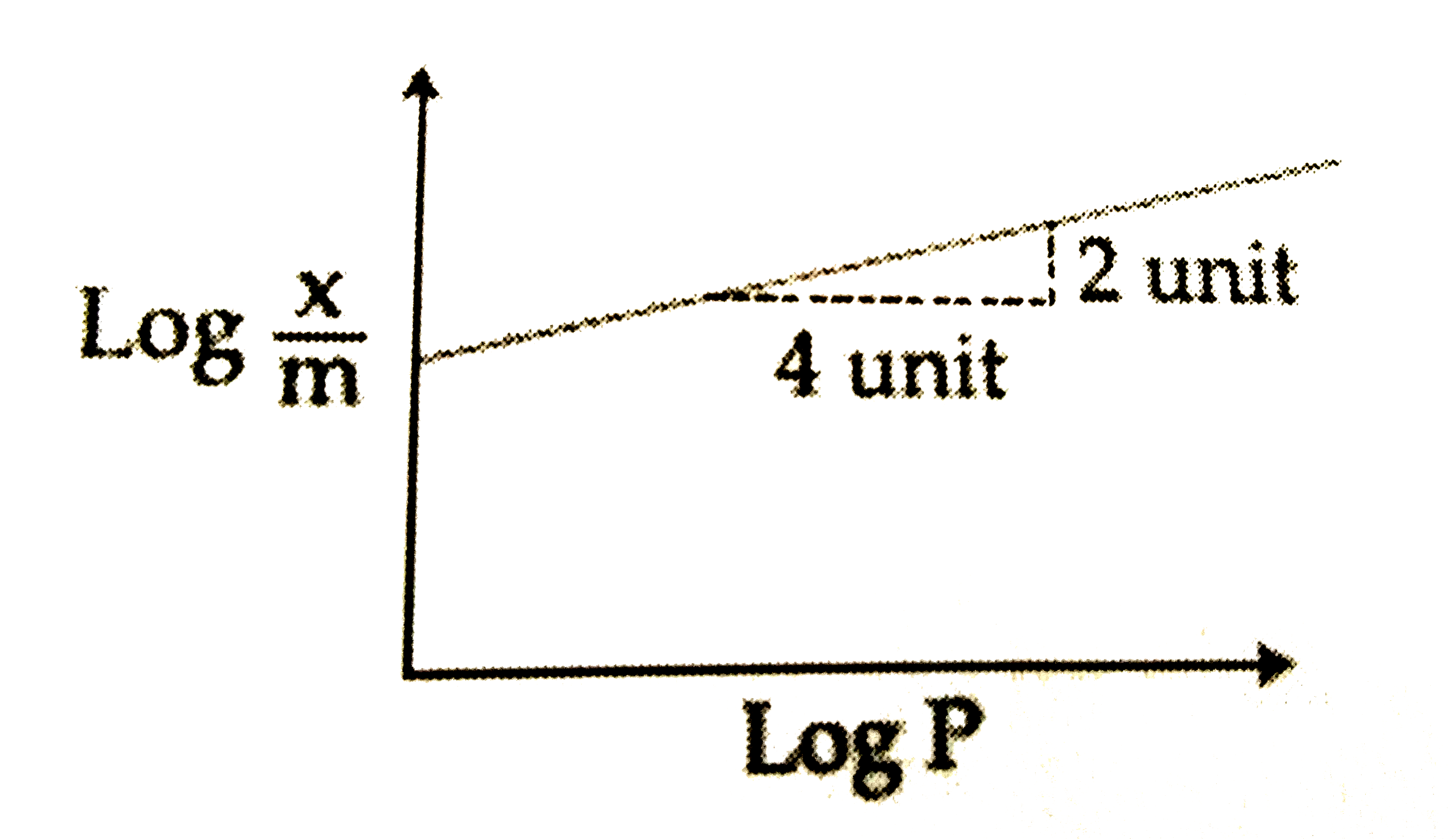 AP14BP2CPDP12
AP14BP2CPDP12Adsorption of gas follows Freundlich adsorption isotherm. If x is the mass of the gas adsorbed on mass m of the adsrobent , the correct plot of xm versus p is :
A B
B C
C D
D
CH4 is adsorbed on 1g charcoal at 0∘C following the Freundlich adsorption isotherm. 10.0 mL of CH4 is adsorbed at 100 mm of Hg, whereas 15.0 mL is adsorbed at 200 mm of Hg. The volume of CH4 adsorbed at 300 mm of Hg is 10xmL. The value of x is _______ ×10−2. (Nearest integer)
[Use log102=0.3010,log103=0.4771]
View SolutionThe difference between bond orders of CO and NO⊕ is x2 where x = _________ .
(Round off to the Nearest Integer)
View SolutionIn the Freundlich adsorption isotherm, the value of x/m is 0.4 under a pressure of 0.2 atm. Calculate the value the intercept.
View SolutionIn a solvent 50% of an acid HA dimerizes and the rest dissociates. The van't Hoff factor of the acid is ___________×10−2.
(Round off to the nearest integer)
(Round off to the nearest integer)
View SolutionAdsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of logxm versus log p is shown in the graph. xm is proportional to:
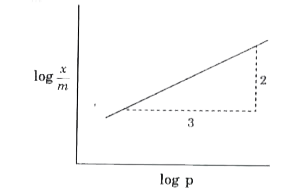
View Solution
