Which pair of atoms can form an ionic bond?
sodium, Na, and potassium, K
potassium, K, and flourine, F
flourine, F, and chlorine, Cl
sodium, Na, and neon, Ne
The correct Answer is:B
To determine which pair of atoms can form an ionic bond, we need to analyze the given pairs of atoms based on their ability to lose or gain electrons. Ionic bonds typically form between metals and non-metals, where the metal loses electrons and the non-metal gains electrons.
1. Identify the pairs of atoms: The question provides pairs of atoms. For this example, we will consider Sodium (Na) and Potassium (K), as well as Potassium (K) and Fluorine (F).
2. Analyze Sodium (Na) and Potassium (K):
- Sodium (Na) has an atomic number of 11 and an electron configuration of 2, 8, 1. It tends to lose one electron to achieve a stable configuration (2, 8).
- Potassium (K) has an atomic number of 19 and an electron configuration of 2, 8, 8, 1. It also tends to lose one electron to achieve a stable configuration (2, 8, 8).
- Since both Sodium and Potassium are metals from the same group (Group 1), they will not form an ionic bond because neither will accept an electron; both will lose one.
3. Analyze Potassium (K) and Fluorine (F):
- Potassium (K) again has an atomic number of 19 and an electron configuration of 2, 8, 8, 1. It will lose one electron to achieve a stable configuration (2, 8, 8).
- Fluorine (F) has an atomic number of 9 and an electron configuration of 2, 7. It needs one electron to complete its outer shell and achieve a stable configuration (2, 8).
- In this case, Potassium will lose one electron, and Fluorine will gain that electron. This transfer of electrons leads to the formation of an ionic bond, resulting in K+ and F- ions.
4. Conclusion: The pair of atoms that can form an ionic bond is Potassium (K) and Fluorine (F).
Final Answer:
The correct pair of atoms that can form an ionic bond is Potassium (K) and Fluorine (F).
- Sodium (Na) has an atomic number of 11 and an electron configuration of 2, 8, 1. It tends to lose one electron to achieve a stable configuration (2, 8).
- Potassium (K) has an atomic number of 19 and an electron configuration of 2, 8, 8, 1. It also tends to lose one electron to achieve a stable configuration (2, 8, 8).
- Since both Sodium and Potassium are metals from the same group (Group 1), they will not form an ionic bond because neither will accept an electron; both will lose one.
- Potassium (K) again has an atomic number of 19 and an electron configuration of 2, 8, 8, 1. It will lose one electron to achieve a stable configuration (2, 8, 8).
- Fluorine (F) has an atomic number of 9 and an electron configuration of 2, 7. It needs one electron to complete its outer shell and achieve a stable configuration (2, 8).
- In this case, Potassium will lose one electron, and Fluorine will gain that electron. This transfer of electrons leads to the formation of an ionic bond, resulting in K+ and F- ions.
The correct pair of atoms that can form an ionic bond is Potassium (K) and Fluorine (F).
Topper's Solved these Questions
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 ( Answer the following questions )
Explore 4 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:SELF ASSESSMENT SHEET -20
Explore 23 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 (FILL IN THE BLANKS WITH APPROPRIATE WORDS TO MAKE CORRECT STATEMENTS. )
Explore 10 Videos
- CARBON AND ITS COMPOUNDS Book:S CHAND IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDS Exercise:SELF ASSESSMENT SHEET-19
Explore 24 Videos
Similar Questions
What particle is needed to begin a nuclear chain reaction?
View SolutionWhat is the maximum number of electrons which can be accomodated in the L shell of an atom ?
View SolutionThe electrons present in the outermost shell of an atom are called_____ electrons.
View SolutionMatch correctly
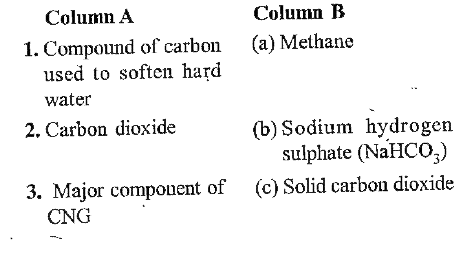
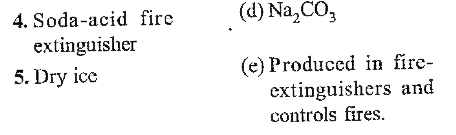
View SolutionThe pair of elements which form ionic bond is .
View SolutionCan ionic bond be formed between similar atoms ?
View SolutionWhich of the following pairs will form the most stable ionic bond ?
View SolutionWhich pair of atoms form strongest ionic bond ?
View SolutionWhich of the following pairs will form the most stable ionic bond ?
View SolutionWhich of the following pairs will form the most stable ionic bond?
View SolutionWhich of the following pairs will form the most stable ionic bond?
View SolutionWhich pair of elements would form the most ionic bond?
View SolutionWhich of the following pairs will form the most stable ionic bond ?
View SolutionWhich pair of atoms form strongest ionic bond ?
View SolutionWhich pair of atoms form strongest ionic bond ?
View SolutionThe pair of elements which form ionic bond is .
View Solution