The functional group of alcohols is
−O∣∣C−O−
−O∣∣C−OH
−OH
−H∣C=O
The correct Answer is:C
Step by step video & text solution for The functional group of alcohols is by Chemistry experts to help you in doubts & scoring excellent marks in Class 9 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CARBON AND ITS COMPOUNDSBook:MTG IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDSExercise:Exercise (Match the Following)
Explore 5 Videos
- CARBON AND ITS COMPOUNDSBook:MTG IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDSExercise:Exercise (Assertion & Reason Type)
Explore 10 Videos
- CARBON AND ITS COMPOUNDSBook:MTG IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDSExercise:Solved Examples
Explore 15 Videos
- ATOMS AND MOLECULES Book:MTG IIT JEE FOUNDATIONChapter:ATOMS AND MOLECULES Exercise:OLYMPIAD/HOTS CORNER
Explore 20 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:EXERCISE ( Integer/Numerical Value Type )
Explore 8 Videos
Similar Questions
Knowledge Check
A functional group is an atom or group of atoms which determines the characteristic functions or chemical properties of a particular organic compound. A functional group contains one or more atoms of elements other than carbon such as oxygen, nitrogen, sulphur and halogens. These atoms are known as heteroatoms and they can replace one or more hydrogen atoms in a hydrocarbon chain. These heteroatoms are responsible for the specific properties of organic compounds regardless of nature and length of carbon chain. These heteroatoms or group of heteroatoms are known as functional groups. The examples of functional groups are - OH, - F,- Cl, -Br, - CHO, - COOH, −NO2,−NH2, etc.
The functional group of alcohol isA−OHB−COOHC−CHOD−ClThe functional isomer of alcohol is
AetherBaldehydeCketoneDcarboxylic acidFuntional group of primary alcohols is
A>CHOHB>C−OHC−CH2OhD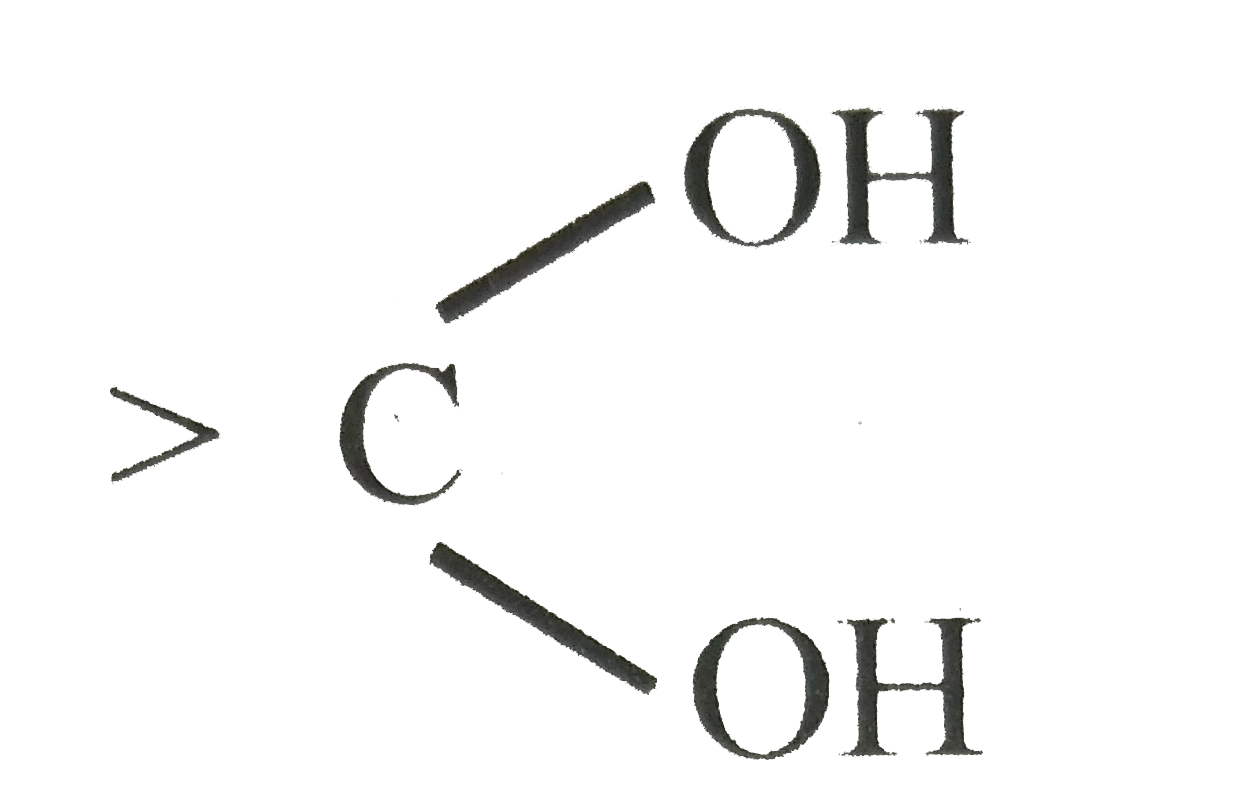
Assertion(A)Lower alcohols are soluble in water.
Reason(R ) There is the dipole-dipole interaction of OH group of alcohols with water molecules.
View Solution(a) Which functional group do you think can be presetn in an organic compound having the molecular formula C5H10O2? Write the formujla of the organic compound.
(b) ive one explae each of the compounds having the following functional groups:
(i) Aldehyde group , (ii) Alcohol gorup ,(iii) Crboxylic acid group ,(iv) Halo group
View SolutionThe functional group present in alcohol is :
View SolutionAssertion : The functional group present in alcohols is -OH.
Reaction : It is the same group as present in water, hence water and alcohol have similar properties.
View SolutionThe function of alcohol addition in stroing chloroform is :
View Solution