A silver ornament of mass 'm' gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament. [At. Mass : silver = 108u, gold = 197u]
Mass of silver = m g
Mass of gold =m100g
Number of atoms of silver =MassAtomic mass×NA=m108×NA
Number of atoms of gold =m100×197×NA
Ratio of number of atoms of gold to silver =Au:Ag
=m100×197×NA:m108×NA
=108:100×197=108:19700=1:182.41
Topper's Solved these Questions
- FOOTSTEPS TOWARDS(CBSE BOARD)BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS(CBSE BOARD)EXERCISE - SECTION-C
15 Videos
- FOOTSTEPS TOWARDS (NEET)BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS (NEET)EXERCISE - QUESTIONS
43 Videos
- IS MATTER AROUND US PURE ?BOOK - MTG IIT JEE FOUNDATIONCHAPTER - IS MATTER AROUND US PURE ?EXERCISE - OLYMPIAD/ HOTS CORNER
20 Videos
Similar Questions
A hot solution contains 5g of a substance in 15g of water at 35∘C. What is the solubility of the substance at this temperature?
View SolutionWhich of the following would be described as impure?
View SolutionThree students Ankit, Dinesh and Manoj were given three unknown substances X, Y and Z respectively during the lab activity.
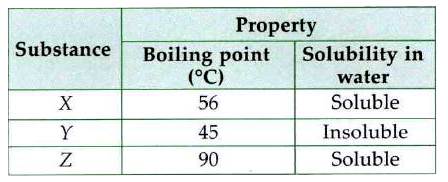
On the basis of these properties, which student has chosen the correct separation technique, to separate a substance from the substance-water mixture?
View SolutionA piece of alloy of gold and silver weighs 2kg in air and 1.86kg in water. What is the mass of silver? Density of gold =19.3×103kgm−3. Density of silver =10.5×1030kgm−3.
View Solutionसोने और चांदी की दो मिश्रधातुओं में सोने और चांदी का अनुपात 7:22 और 21:37 है इन दोनों मिश्रधातुओं को किस अनुपात में मिलाया जाए ताकि नयी मिश्रधातु में सोने और चांदी का अनुपात 25:62 हो !
View SolutionA silver ornament of mass 'm' gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
View SolutionThe same quantity of electricity that liberated 2.158 g silver was passed through a solution of a gold salt and 1.314 g of gold was deposited. The equivalent mass of silver is 107.9. Calculate the equivalent mass of gold. What is the oxidation state of gold salt ? (At. mass of gold =197)
View SolutionSilver plated spoon, gold plated ornaments are the examples of alloying True/False
View SolutionSilver plated spoon, gold plated ornaments are the examples of alloying.
View SolutionAn alloy contains gold and silver in the ratio 5 : 8 and another alloy contains gold and silver in the ratio 5 : 3. If equal amount of both the alloys are melted together, then the ratio of gold and silver in the resulting alloy is ?
View SolutionA gold crown adulterated with silver was found to weigh 0.540 kg in air and 0.498 kg in water. If the densities of gold and silver are 13900kgm−3and10500kgm−3, then calculate the mass of silver mixed with gold.
View Solutionಕಾರಣಕೊಡಿ ಪ್ಲಾಟಿನಂ, ಚಿನ್ನ ಮತ್ತು ಬೆಳ್ಳಿಯನ್ನು ಆಭರಣಗಳ ತಯಾರಿಕೆಯಲ್ಲಿ ಬಳಸುವರು.
View SolutionAlloy A contains 40% gold and 60% silver. Alloy B contains 35% gold and 40% silver and 25% copper. Alloys A and B are mixed in the ratio of 1:4. What is the ratio of gold and silver in the newly formed alloy is?
View SolutionA silver ornament of mass 'm' gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
View Solutionm,gm द्रव्यमान के एक सिल्वर के आभूषण को सिल्वर के 1% द्रव्यमान तुल्य गोल्ड से पोलिश किया गया । आभूषण में गोल्ड और सिल्वर के परमाणुओं की संख्याओं के अनुपात को परिकलित कीजिए।
View SolutionWhich of the following acid is used by goldsmiths for cleaning gold and silver ornaments?
View Solution
