Elements from A to F have in them the distribution of electrons, neutrons and protons as follows:
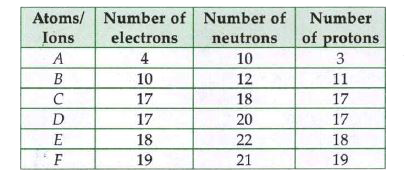
Making use of these data, find
a pair of isobars
A pair of isobars is E and F.
Mass number of E = 22 + 18 = 40 Mass number of F = 21 + 19 = 40
Topper's Solved these Questions
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE (Multiple Choice Questions)
Explore 50 Videos
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE (Match the following)
Explore 6 Videos
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE ( Integer/Numerical Value Type)
Explore 5 Videos
- COAL AND PETROLEUMBook:MTG IIT JEE FOUNDATIONChapter:COAL AND PETROLEUMExercise:OLYMPIAD/HOTS CORNER
Explore 10 Videos
Similar Questions
Elements from A to F have in them the distribution of electrons, neutrons and protons as follows:
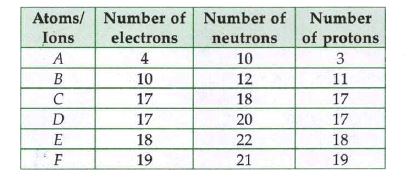
Making use of these data, find
a pair of ions
View SolutionElements from A to F have in them the distribution of electrons, neutrons and protons as follows:
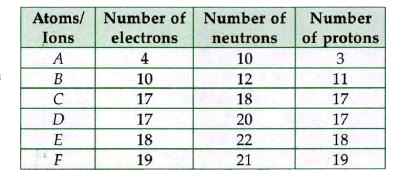
Making use of these data, find
a pair of isotopes.
View Solution
Knowledge Check
The charge on the atom having 17 protons, 18 neutrons and 18 electrons is
A+1B−1C−2DZeroThe charge on the atom containing 17 protons, 18 neutrons and 18 electrons is.
A+1B−1C−2DZero
Elements from A to F have in them the distribution of electrons, neutrons and protons as follows:
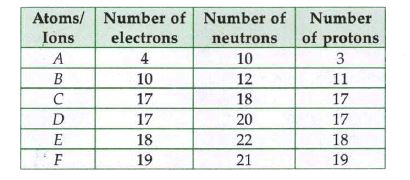
Making use of these data, find
an atom of a noble gas
View SolutionState the number of protons electrons and neutrons in the following :
Ca
Ne
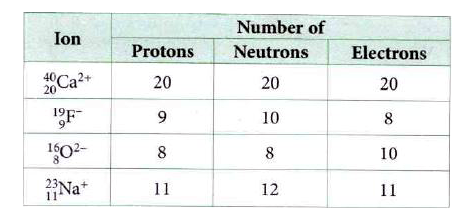
View SolutionThe table given shows the number of protons, neutrons and electrons in four ions. For which ion is the data correct?
View SolutionUse the following terms in the same sentence proton, neutron, and isotope.
View SolutionThe atoms of all the elements contains electrons, protons and neutrons. True/False explain.
View SolutionThe atom of an element has 9 protons, 9 electrons and 10 neutrons.
What is the mass number of the element?
View Solution