The percentage composition of elements in a compound is calculated from the molecular formula of the compound. The molecular mass of a compound is calculated from the atomic masses of the various elements present in the compound. The percentage of mass of each element is then calculated with the help of the following relation. Percentage mass of the element in the compound.
Mass of the elementMass of the compound
Percentage of S in aluminium sulphate is
28
56
65
75
The correct Answer is:A
To calculate the percentage of sulfur (S) in aluminum sulfate (Al₂(SO₄)₃), we will follow these steps:
Step 1: Determine the Molecular Formula
The molecular formula of aluminum sulfate is Al₂(SO₄)₃.
Step 2: Calculate the Molar Mass of the Compound
To find the molar mass of aluminum sulfate, we need to sum up the atomic masses of all the elements in the formula:
- Aluminum (Al): The atomic mass of aluminum is approximately 27 g/mol. Since there are 2 aluminum atoms:
Mass of Al=2×27=54 g/mol
- Sulfur (S): The atomic mass of sulfur is approximately 32 g/mol. Since there are 3 sulfur atoms:
Mass of S=3×32=96 g/mol
- Oxygen (O): The atomic mass of oxygen is approximately 16 g/mol. Since there are 12 oxygen atoms (4 from each sulfate group and there are 3 sulfate groups):
Mass of O=12×16=192 g/mol
Now, we can calculate the total molar mass of aluminum sulfate:
Molar Mass of Al₂(SO₄)₃=Mass of Al+Mass of S+Mass of O
=54+96+192=342 g/mol
Step 3: Calculate the Percentage of Sulfur
Now, we can calculate the percentage of sulfur in aluminum sulfate using the formula:
Percentage of S=(Mass of SMass of compound)×100
Substituting the values:
Percentage of S=(96342)×100
Calculating this gives:
Percentage of S≈28.07%
Final Answer
The percentage of sulfur in aluminum sulfate is approximately 28%.
---
The molecular formula of aluminum sulfate is Al₂(SO₄)₃.
To find the molar mass of aluminum sulfate, we need to sum up the atomic masses of all the elements in the formula:
Mass of Al=2×27=54 g/mol
Mass of S=3×32=96 g/mol
Mass of O=12×16=192 g/mol
Molar Mass of Al₂(SO₄)₃=Mass of Al+Mass of S+Mass of O
=54+96+192=342 g/mol
Now, we can calculate the percentage of sulfur in aluminum sulfate using the formula:
Percentage of S=(Mass of SMass of compound)×100
Substituting the values:
Percentage of S=(96342)×100
Calculating this gives:
Percentage of S≈28.07%
The percentage of sulfur in aluminum sulfate is approximately 28%.
Topper's Solved these Questions
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE ( Comprehension Type PASSAGE-II )
Explore 4 Videos
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE ( Comprehension Type PASSAGE-III )
Explore 3 Videos
- ATOMS, MOLECULES AND ATOMIC STRUCTUREBook:MTG IIT JEE FOUNDATIONChapter:ATOMS, MOLECULES AND ATOMIC STRUCTUREExercise:EXERCISE (Assertion & Reason Type)
Explore 10 Videos
- COAL AND PETROLEUMBook:MTG IIT JEE FOUNDATIONChapter:COAL AND PETROLEUMExercise:OLYMPIAD/HOTS CORNER
Explore 10 Videos
Similar Questions
In chemistry, 'mole' is an essential tool for the chemical calculations. It is a basic SI unit adopted by the 14th general conference on weights and measurements in 1971. A mole contains as many elementary particles as the number of atoms present in 12 g of 12C . 1 mole of a gas at STP occupies 22.4 litre volume. Molar volume of solids and liquids is not definite. Molar mass of a substance is also called gram-atomic mass or gram molecular mass. The virtual meaning of mole is plenty, heap or the collection of large numbers. 1 mole of a substance contains 6.022×1023 elementary particles like atom or molecule. Atomic mass unit (amu) is the unit of atomic mass, e.g., atomic mass of single carbon is 12 amu.
The mass of one molecule of water is approximately
View SolutionAccording to Dalton's atomic theory, all matter whether an element, a compound or a mixture is composed of small particles called atoms which can neither be created nor destroyed during a chemical reaction. Dalton's theory provides a simple explanation for the laws of chemical combination. He used his theory to explain law of conservation of masses, law of constant proportions and law of multiple proportions, based on various postulates of the theory. Dalton was the first scientist to use the symbols for the elements in a very specific sense. When he used a symbol for an element he also meant a definite quantity of that element, that is one atom of that element.
Which postulate of Dalton's atomic theory is the result of the law of conservation of mass?
View SolutionAssertion : Number of gram-molecules of SO2Cl2 in 13.5 g of sulphuryl chloride is 0.2.
Reason : Gram-molecular mass of SO2Cl2 is 125 g.
View SolutionFill in the blanks left in the classification of the fossil fuels.
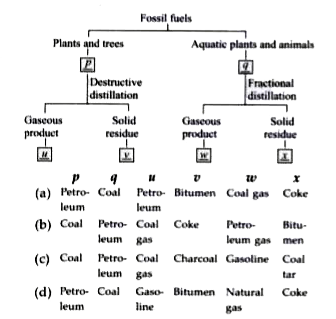
View SolutionA crystalline compound when heated become anhydrous by losing 51.2 % of the mass. On analysis, the anhydrous compound gave the following percentage composition :
Mg=20.0%,S=26.66%andO=53.33%. Calculate the molecular formula of the anhydrous compound and crystalline compound. The molecular mass of anhydride compound is 120 u.
View SolutionA compound (molecular mass 64.59 u) has the following data : {:("Element","Relative No. of atoms","% Composition"),("A",3.10,37.215),("B",7.80,7.8),("C",1.55,54.98):} Determine : (i) Atomic masses of the elements A, B, C (ii) Simplest atomic ratios of the element (iii) Molecular formula of the compound.
View SolutionEmpirical formula is the simplest formula of the compound which gives the atomic ratio of various elements present in one molecule of the compound. However, the molecular formula of the compound gives the number of atoms of various elements present in one molecule of the compound.
Molecular formula=(Empirical formula)×n
n=Molecular massEmpirical formula mass
A compound may have same empirical and molecular formulae Both these formulae are calculated by using percentage composition of constituent elements.
Which of the following compounds have same empirical formula?
View SolutionEmpirical formula is the simplest formula of the compound which gives the atomic ratio of various elements present in one molecule of the compound. However, the molecular formula of the compound gives the number of atoms of various elements present in one molecule of the compound.
Molecular formula=(Empirical formula)×n
n=Molecular massEmpirical formula mass
A compound may have same empirical and molecular formulae Both these formulae are calculated by using percentage composition of constituent elements.
Which of the following represents the formula of a substance which contains 50% oxygen?
View SolutionEmpirical formula is the simplest formula of the compound which gives the atomic ratio of various elements present in one molecule of the compound. However, the molecular formula of the compound gives the number of atoms of various elements present in one molecule of the compound. Molecular formula=(Empirical formula) xxn n=("Molecular mass")/("Empirical formula mass") A compound may have same empirical and molecular formulae Both these formulae are calculated by using percentage composition of constituent elements. The pair of species having different percentage composition of carbon are:
View SolutionEmpirical formula is the simplest formula of the compound which gives the atomic ratio of various elements present in one molecule of the compound. However, the molecular formula of the compound gives the number of atoms of various elements present in one molecule of the compound. Molecular formula=(Empirical formula) xxn n=("Molecular mass")/("Empirical formula mass") A compound may have same empirical and molecular formulae Both these formulae are calculated by using percentage composition of constituent elements. A compound of Na, C and O contains 0.0887 mol Na, 0.132 mol O and 2.65xx10^(22) atoms of carbon . the empirical formula of the compound is:
View SolutionThe simplest formula of a compound containing 50% of element X (atomic mass 10) and 50% of element Y (atomic mass 20) is
View SolutionA compound has the following percentage composition. Carbon 80%, Hydrogen 20%? If the molecular mass is 30, Calculate the molecular formula.
View SolutionA crystalline compound when heated became anhydrous by losing 51.2 % of the mass. On analysis, the anhydrous compound gave the following percentage composition: Mg = 20.0 %, S = 26.66 % and O = 53.33 %, Calculate the molecular formula of the anhydrous compound and crystalline compound. The molecular mass of anhydrous compound is 120 u.
View Solutionपारे व क्लोरीन के एक यौगिक में इन तत्वों की प्रतिशत मात्रा 84.9 व 15.1 है। यदि यौगिक का अणु द्रव्यमान 471 हो, तो उसका आण्विक सूत्र ज्ञात कीजिए। (Hg = 200, Cl = 35.5)
View SolutionThe simplest formula of a compound containing 50 % of element X (atomic mass 10 ) and 50 % of element Y (atomic mass 20 ) is
View SolutionA crystalline compound when heated became anhydrous by losing 51.2 % of the mass. On analysis, the anhydrous compound gave the following percentage composition: Mg = 20.0 %, S = 26.66 % and O = 53.33 %, Calculate the molecular formula of the anhydrous compound and crystalline compound. The molecular mass of anhydrous compound is 120 u.
View Solution