Magnesium powder was added to two different salt solutions in beakers (I) and (II) as shown in the given figures. Answer the questions that follow:
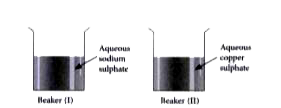 What would you observe when magnesium powder is added to beaker (I)? Explain your answer.
What would you observe when magnesium powder is added to beaker (I)? Explain your answer.
No change will be observed when magnesium powder is added to beaker (I) because magnesium being less reactive than sodium cannot displace sodium from its solution.
Topper's Solved these Questions
- FOOTSTEPS towards(CBSE Board)BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS towards(CBSE Board)EXERCISE - SECTION - C
11 Videos
- FOOTSTEPS TOWARDS (NEET)BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS (NEET)EXERCISE - Multiple Choice Question
45 Videos
- MATERIALS : METALS AND NON-METALSBOOK - MTG IIT JEE FOUNDATIONCHAPTER - MATERIALS : METALS AND NON-METALSEXERCISE - Olympiad/HOTS Corner
20 Videos
Similar Questions
Write three differences between natural and synthetic fibres. Give two examples of each.
View SolutionThe synthetic material which can be used for making fabrics as well as shatterproof bottles and jars is
View SolutionWhen a copper vessel is exposed to moist air for long, it acquires a dull green coating. The reaction can be represented as
2Cu+W+X+Ymoist air→Cu(OH)2+Z
W,X,Y and Z respectively
View SolutionPrachi took 50 mL of water in two beakers at room temperature and added sodium chloride to one beaker while sugar to the other, till no more solute would dissolve. Then she heated the contents of the beakers and added more solutes in them.
(a) Will the amount of salt and sugar that can be dissolved in water at given temperature same ?
(b) What will you expect to happen if she cools the contents of the beakers ? Justify your answer.
View SolutionA student mixes sodium sulphate powder in barium chloride powder. What change would the student observe on mixing the two powders? Justify your answer and explain how he can obtain the desired change.
View SolutionObserve the figure and answer the following questions. (i) Label (A) to (D) (ii) What process is shown in the diagram ? (iii) Explain the process
View Solutionमैग्नीशियम पाउडर वायु में जलकर देता है
View Solutionमैग्नीशियम पाउडर वायु में जलकर देता है
View SolutionA student mixes sodium sulphate powder in barium chloride powder. What change would the student observe on mixing the two powders? Justify your answer and explain how he can obtain the desired change.
View SolutionGive your observation : Magnesium powder is dropped in very dilute solution of nitric acid.
View SolutionState your observation : When sodium hydroxide is added to magnesium nitrate solution
View SolutionState your observation when Ammonium hydroxide solution is added to magnesium chloride
View SolutionDrop an egg in a beaker of water. Now drop the same egg in another beaker of water in which excessive salt is added. Write your observation.
View SolutionA little distilled water is taken in a beaker.
A) What is the pH of distilled water?
B) What happens to the pH value when the following substances are added to the water in the beaker? Justify your answer.
a) Caustic soda
b) Vinegar
View SolutionA little distilled water is taken in a beaker.
A) What is the pH of distilled water?
B) What happens to the pH value when the following substances are added to the water in the beaker? Justify your answer.
a) Caustic potash
b) Lemon juice
View Solution
