In the following situations, a reaction may or may not take place. If it does, write the balanced molecular, total ionic and net ionic equations illustrating the reaction. Assume all the reactions take place in aqueous solutions.
Some iron nails are placed in a CuCl2 solution.
Molecular equation:
CuCl2(aq)+Fe(s)→FeCl2(aq)+Cu(s)
Total ionic equation :
Cu2+(aq)+2Cl−(aq)+Fe(s)→Fe2+(aq)+2Cl−(aq)+Cu(s)
Net ionic equation :
Cu2+(aq)+Fe(s)→Fe2+(aq)+Cu(s)
टॉपर्स ने हल किए ये सवाल
- CHEMICAL REACTIONS AND EQUATIONSBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:EXERCISE (LONG ANSWER TYPE)
Explore 34 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:EXERCISE (INTEGER/NUMERICAL VALUE TYPE)
Explore 5 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:EXERCISE (VERY SHORT ANSWER TYPE)
Explore 16 Videos
- CHEMICAL EQUILIBRIUMBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL EQUILIBRIUMExercise:EXERCISE (INTEGER/NUMERICAL VALUE TYPE)
Explore 5 Videos
- FOOTSTEPS TOWARDS (NEET) Book:MTG IIT JEE FOUNDATIONChapter:FOOTSTEPS TOWARDS (NEET) Exercise:MCQ
Explore 45 Videos
समान प्रश्न
In the following situations, a reaction may or may not take place. If it does, write the balanced molecular, total ionic and net ionic equations illustrating the reaction. Assume all the reactions take place in aqueous solutions.
A copper wire is placed in Pb(NO3)2 solution.
View SolutionIn the following situations, a reaction may or may not take place. If it does, write the balanced molecular, total ionic and net ionic equations illustrating the reaction. Assume all the reactions take place in aqueous solutions.
Silver coins are dropped in hydrochloric acid solution.
View Solution
ज्ञान जांच
Ionic reactions takes place in
ALiquid stateBSolid stateCSolution stateDGaseous stateHaloform reaction does not take place with
AacetoneB2-chloropropaneCethanolDmethanolCannizzaro reaction does not take place with
A(CH3)3CCHOB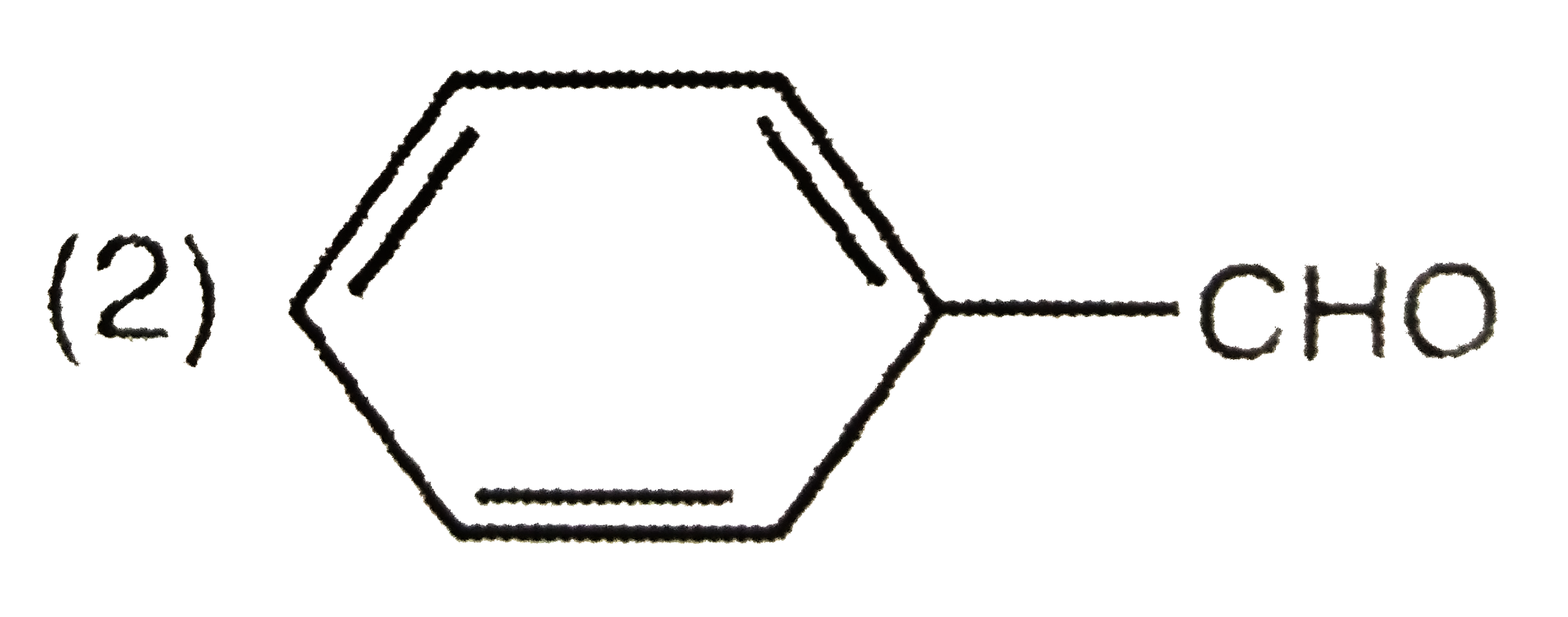 C
C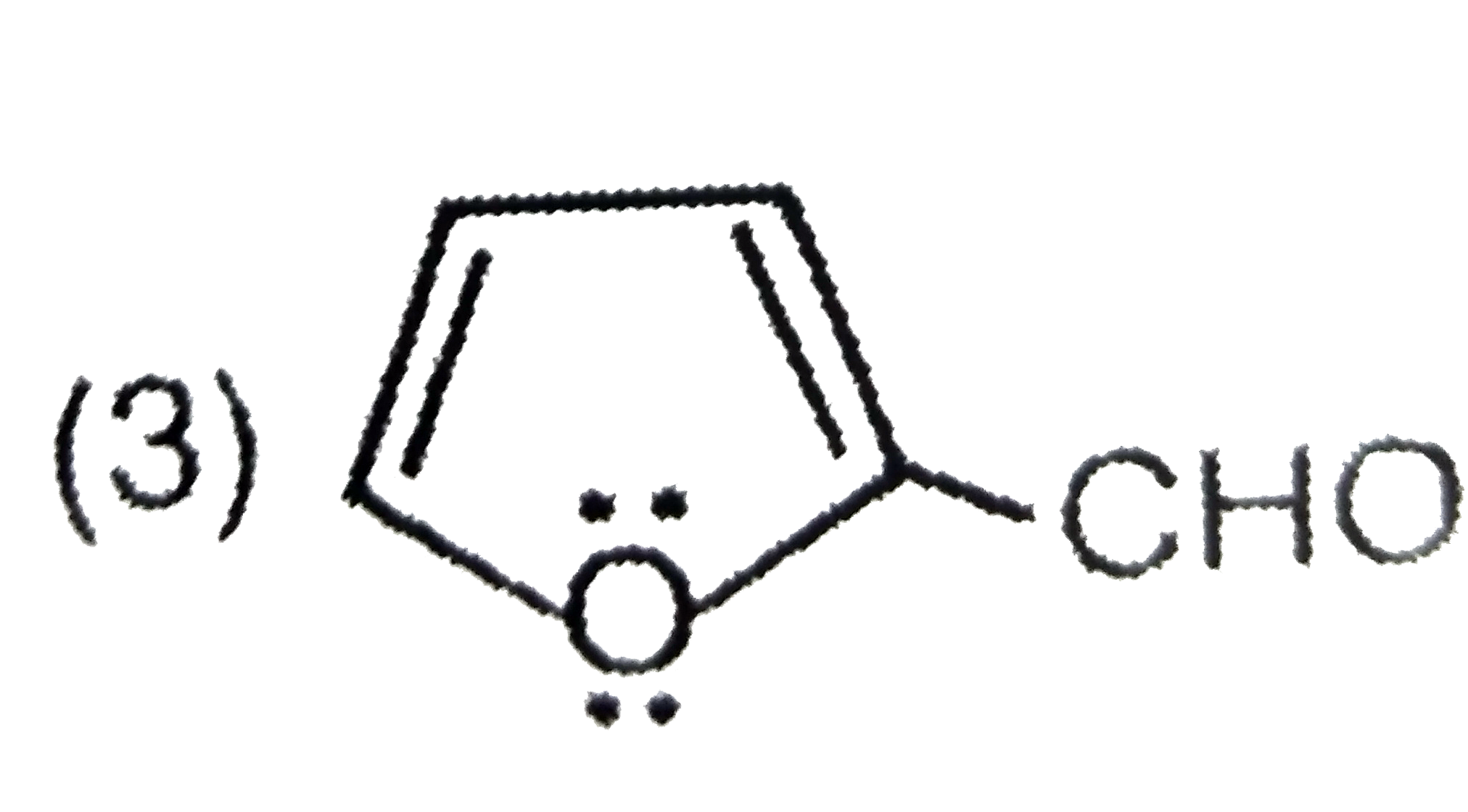 DCH3CHO
DCH3CHO
Give one example of a reversible reaction taking place in aqueous solution.
View SolutionHaloform reaction does not take place with :
View SolutionIn which of the following case disproportionatlon reaction take place.
View SolutionIn which of the following reaction an acid-base reaction takes place ?
View SolutionWhich of the following reaction does not take place at cathode
View Solution
