The given diagram shows the energy levels of the reactants and products for a particular reaction:

Which of the following processes can be related to the given diagram?
Ethyne gas burns in oxygen to form carbon dioxide and water along with evolution of heat.
When solid mercury (II) oxide is heated liquid mercury and oxygen gas are produced.
Hydrogen gas combines with chlorine gas in the presence of light to form hydrogen chloride gas.
Potassium chlorate decomposes in presence of heat to form potassium chloride and oxygen.
The correct Answer is:A
In the given diagram, the energy of reactants is greater than products, thus, energy is released during the reaction i.e., the reaction is exothermic.
Ethyne gas burns in oxygen as:
C2H2Ethyne+52O2Oxygen→2CO2Carbondioxide+H2OWater+Heat
Whereas, all other given reactions consume heat or light.
Topper's Solved these Questions
- CHEMICAL REACTIONS AND EQUATIONSBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:EXERCISE (INTEGER/NUMERICAL VALUE TYPE)
Explore 5 Videos
- CHEMICAL EQUILIBRIUMBook:MTG IIT JEE FOUNDATIONChapter:CHEMICAL EQUILIBRIUMExercise:EXERCISE (INTEGER/NUMERICAL VALUE TYPE)
Explore 5 Videos
- FOOTSTEPS TOWARDS (NEET) Book:MTG IIT JEE FOUNDATIONChapter:FOOTSTEPS TOWARDS (NEET) Exercise:MCQ
Explore 45 Videos
Similar Questions
The value of x in balanced equation is
C6H12O6(aq)+6O2(g)→xCO2(g)+6H2O(l)+energy
View SolutionFor the reaction :
2A→B+C
At a given time, the concentration of reaction mixture is [A]=[B]=[C]=3×10−4M. The value of reaction quotient for the reaction is'
View SolutionThe incorrect statement about metals is
View SolutionThe given diagram shows a cell
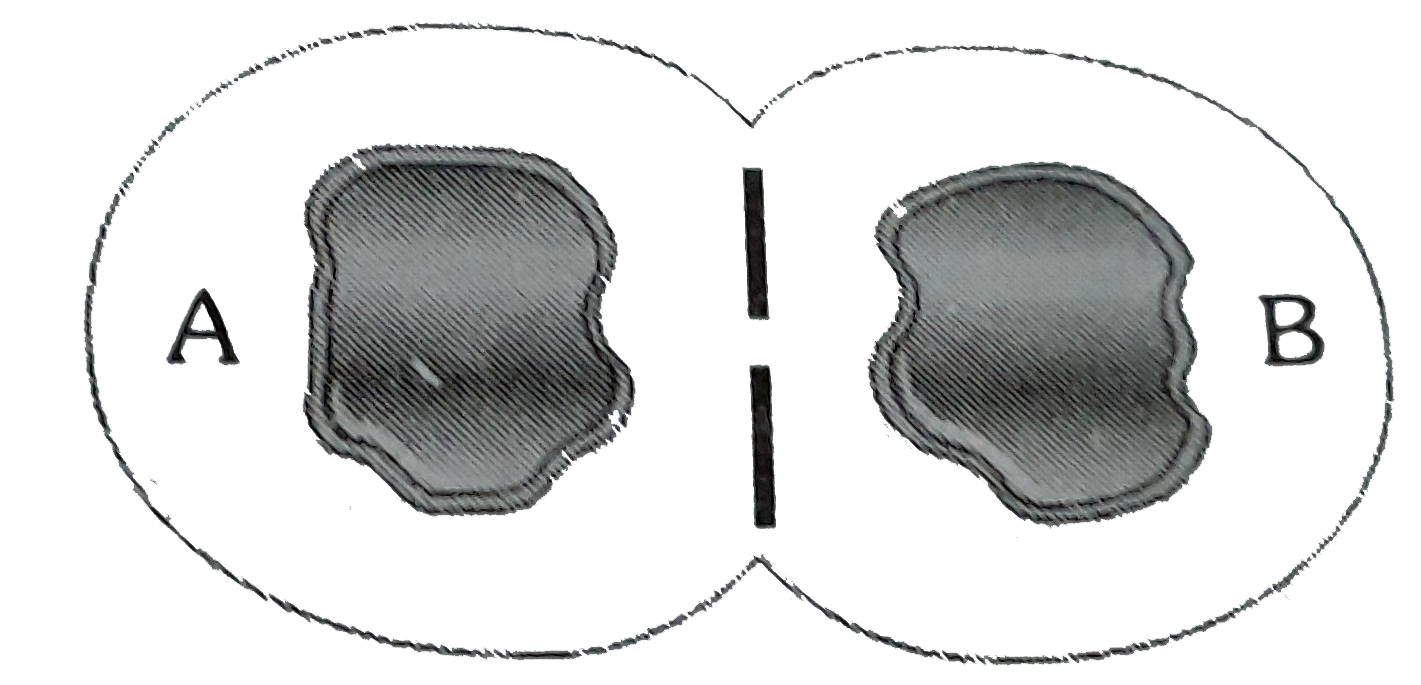
Which of the following statement related to the image is not correct.
View SolutionWhich energy diagram best reprsents the given reaction ?
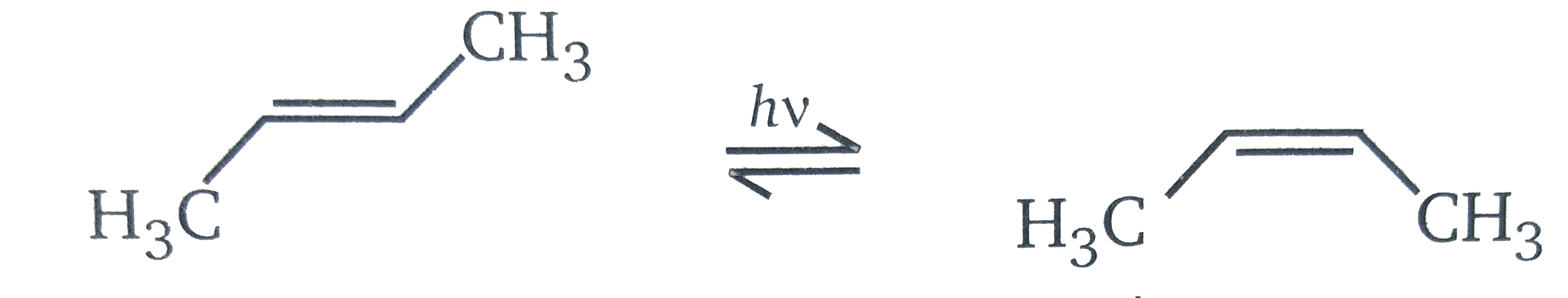
View SolutionEnthalpy diagram for a particular reaction is given in figure. Is it possible to decide spontaneity of a reaction from given diagram. Explain.
View SolutionEnthalpy diagram for a particular reaction is given in figure. Is it possible to decide spontaneity of a reaction from given diagram. Explain
View SolutionConsider the following energy level diagram:
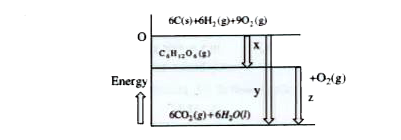
Answer the following question on the basis of the given diagram
The heat of formation of glucose is
View SolutionConsider the following energy level diagram: Answer the following question on the basis of the given diagram In the given diagram z refers to
View SolutionConsider the following energy level diagram: Answer the following question on the basis of the given diagram The quantity y is equal to
View SolutionConsdier the following energy level diagram:
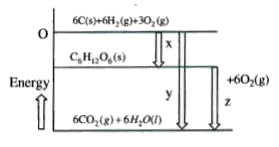
In the given diagram z refer to
View SolutionEnthalpy diagram for a particular reaction is given in figure. Is it possible to decide spontaneity of a reaction from given diagram. Explain.
View Solutionदिए गए गए चित्र में एक पिरैमिड में विभिन्न पोषी स्तर दिखाए गए हैं। बताइए कि किस पोषी स्तर पर सबसे अधिक ऊर्जा उपलब्ध होती है?
View SolutionGiven below is a diagram showing particular type of reproduction. Name this process of reproduction.
View SolutionGiven below is a diagram showing particular type of reproduction.
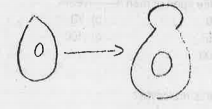
Briefly explain the process.
View Solution
