Arrange the pH in increasing order of hydrogen ion concentration .
লিখিত জবাব
pH in the increasing order of hydrogen ion concentration : pH=11<pH=9<pH=7<pH=4<pH=1
|
টপার এই প্রশ্নগুলো সমাধান করেছে
- ACIDS , BASES AND SALTS Book:MTG IIT JEE FOUNDATIONChapter:ACIDS , BASES AND SALTS Exercise:Exercise (Multiple Choice Questions) LEVEL -1
Explore 30 Videos
- ACIDS , BASES AND SALTS Book:MTG IIT JEE FOUNDATIONChapter:ACIDS , BASES AND SALTS Exercise:Exercise (Multiple Choice Questions) LEVEL -2
Explore 20 Videos
- ACIDS , BASES AND SALTS Book:MTG IIT JEE FOUNDATIONChapter:ACIDS , BASES AND SALTS Exercise:SOLVED EXAMPLES
Explore 20 Videos
- ATOMIC STRUCTURE Book:MTG IIT JEE FOUNDATIONChapter:ATOMIC STRUCTURE Exercise:Exercise (Integer/Numerical Value Type)
Explore 5 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Arrange the following in increasing order of heat of hydrogenation :
 AP>Q>R>SBQ>P>S>RCR>S>Q>PDQ>S>R>P
AP>Q>R>SBQ>P>S>RCR>S>Q>PDQ>S>R>PArrange the following in increasing order of heat of hydrogenation :
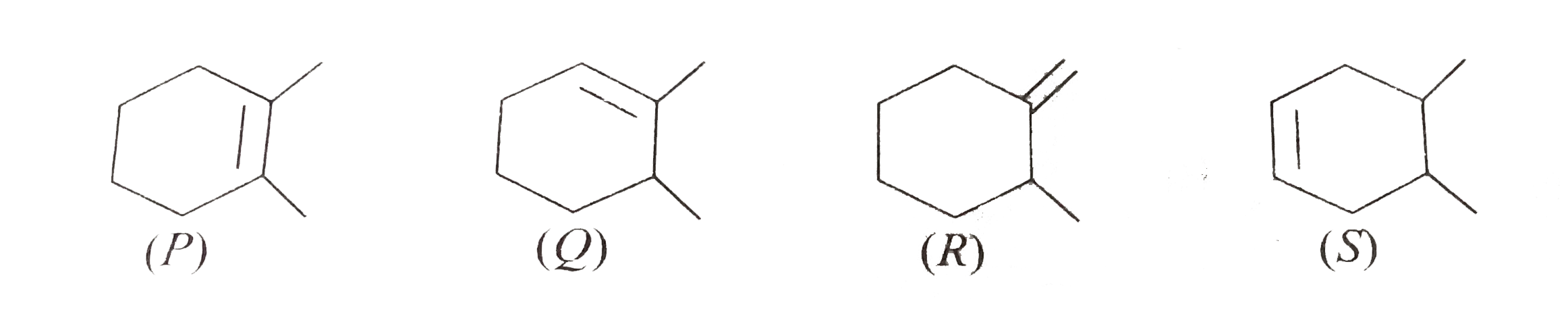 AP>Q>R>SBQ>P>S>RCR>S>Q>TDQ>S>R>P
AP>Q>R>SBQ>P>S>RCR>S>Q>TDQ>S>R>PThe pH of a solution is 6. Sufficient amount of acid is added to decrease the pH to 2. The increase in hydrogen ion concentration is
Athree timesBhundred timesCthousand timesDten thousand times.
The pH of a solution is 5.0. Sufficient acid is added to the solution to decrease the pH to 2.0. The increase in hydrogen ion concentration is
View SolutionIn a solution of pH = 5, more acid is added in order to reduce the pH = 2. The increase in hydrogen ions concentration is
View SolutionIn a solution of pH = 5, more acid is added in order to reduce the pH = 2. The increase in hydrogen ions concentration is
View SolutionpH of different solutions are given in the table
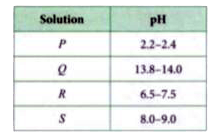
Arrange these solutions in the increasing order of OH− ion concentration .
View Solution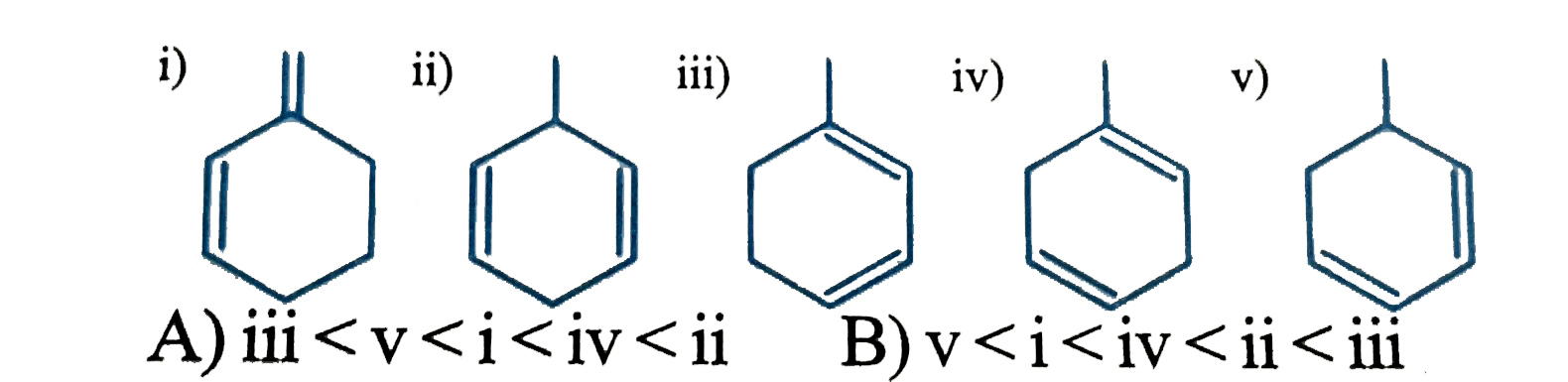
Arrange in increasing order of heat of hydrogenation of following dienes.
View Solution
