Table given below shows a part of the periodic table

Using this table explain why
Atomic size of Mg is less than that of Na
Video Solution
Text Solution
In a period, nuclear charge increases which leads to increased attraction between nucleus and the electrons resulting in decreases in size.
|
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - MTG IIT JEE FOUNDATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - Exercise (Subjective Problems) (Long Answer Type)
13 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - MTG IIT JEE FOUNDATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - Exercise (Integer/Numerical Value Type)
5 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - MTG IIT JEE FOUNDATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - Exercise (Subjective Problems Very Short Answer Type)
26 Videos
- MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES BOOK - MTG IIT JEE FOUNDATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES EXERCISE - EXERCISE (INTEGER/NUMERICAL VALUE TYPE)
5 Videos
Similar Questions
Knowledge Check
The given table shows a part of the periodic table
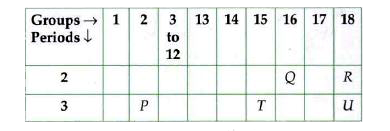
P, Q, R, T and U are respectivelyAMg, S, Ar, Al and NeBO,Mg, Ar, P and NeCMg, O, Ne, P and ArDO, Mg, Ne, P and ArNa belongs to which block in the periodic table ?
As-block,Bp-blockCd-blockDNone of theseThe cation having the largest size in the periodic table is
ABa+2BCa+2CCs+DFe+3
Explain the merits of Mendeleev's periodic table using the following keywords-
A. Atomic Mass
View SolutionGallium lies below aluminium in group 13 of the periodic table but its atomic radius is smaller than that of aluminium. Why so ?
View SolutionIn third period of the long form of the periodic table that is from Na to Cl
View SolutionIn the periodic table , the element with atomic number 38 belongs to ______
View SolutionGa is below Al in the periodic table, but atomic radius of Ga is less than Al. It is because of
View Solution


