Which of the following is independent of temperature?
normality
molarity
molality
All of above
The correct Answer is:C
To determine which concentration term is independent of temperature, we need to analyze the definitions of the concentration terms provided.
1. Understand the Concentration Terms:
- Concentration terms include normality, molarity, molality, and others. Each of these terms has a specific definition related to the amount of solute and the volume or mass of the solution or solvent.
2. Identify the Relationship with Volume:
- Concentration terms that involve volume (like molarity and normality) are affected by temperature changes because temperature can cause the volume of a liquid to expand or contract.
- For example, molarity is defined as moles of solute per liter of solution (which involves volume), and normality is defined as equivalents of solute per liter of solution (also volume-dependent).
3. Examine Molality:
- Molality, on the other hand, is defined as the number of moles of solute per kilogram of solvent. This definition does not involve the volume of the solution, but rather the mass of the solvent.
- Since mass does not change with temperature, molality remains constant regardless of temperature changes.
4. Conclusion:
- Therefore, the concentration term that is independent of temperature is molality.
Final Answer:
Molality is independent of temperature.
- Concentration terms include normality, molarity, molality, and others. Each of these terms has a specific definition related to the amount of solute and the volume or mass of the solution or solvent.
- Concentration terms that involve volume (like molarity and normality) are affected by temperature changes because temperature can cause the volume of a liquid to expand or contract.
- For example, molarity is defined as moles of solute per liter of solution (which involves volume), and normality is defined as equivalents of solute per liter of solution (also volume-dependent).
- Molality, on the other hand, is defined as the number of moles of solute per kilogram of solvent. This definition does not involve the volume of the solution, but rather the mass of the solvent.
- Since mass does not change with temperature, molality remains constant regardless of temperature changes.
- Therefore, the concentration term that is independent of temperature is molality.
Molality is independent of temperature.
টপার এই প্রশ্নগুলো সমাধান করেছে
- MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES BOOK - MTG IIT JEE FOUNDATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES EXERCISE - EXERCISE (MATCH THE FOLLOWING)
5 Videos
- MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES BOOK - MTG IIT JEE FOUNDATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES EXERCISE - EXERCISE (ASSERTION & REASON TYPE)
10 Videos
- MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES BOOK - MTG IIT JEE FOUNDATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES EXERCISE - SOLVED EXAMPLES
13 Videos
- METALS AND NON METALS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - METALS AND NON METALS EXERCISE - Olympiad/HOTS Corner
18 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - MTG IIT JEE FOUNDATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - Olympiad/HOTS Corner
15 Videos
সম্পর্কিত প্রশ্ন
List-IList-II(P)Charles' law(1)V∝n(Q)Boyle's law(2)P∝T(R)Avogadro's law(3)P∝1/V(S)Gay Lussac's law(4)V∝T
View SolutionAssertion : The empirical mass of ethene is half of its molecular mass.
Reason : The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
View SolutionWhy do atomic masses of most of the elements in atomic mass units involve fractions?
View SolutionMetals are extracted from their ores by different methods depending on their position in the activity series
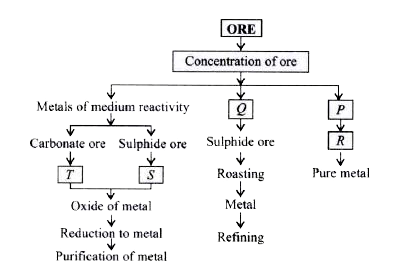
Match the Column-I with Column-II and select the correct option from the codes given below.
Column-I Column-II(A) Highly reactive metals(i) S (B) Calcination(ii) T (C) Electrolytic reduction(iii) P(D) Less reactive metal(iv) Q(E) Roasting(V) Q
View SolutionThe positions of four elements K, L, M and N in the periodic table are shown below:

Which of the following statements are correct?
I. K, L, M and N are metalloids
II. K is a metal while L, M and N are non-metals
III. Among these four elements, K is the smallest in size.
IV. K is a metal while L and M are metalloids and N is a non-metal.
View SolutionWhich of the following concentration terms is independent on temperature ?
View SolutionWhich of the following concentration terms is/are independent of temperature ?
View SolutionWhich of the following terms is independent of temperature ?
View SolutionWhich of the following modes of expressing concentration is independent of temperature
View SolutionWhich of the following terms is independent of temperature ?
View SolutionWhich of the following is temperature independent
View SolutionWhich of the following concentration terms is/are independent of temperature ?
View SolutionWhich of the following concentration terms is/are independent of temperature?
View SolutionWhich of the following concentration terms is/are independent of temperature ?
View SolutionWhich of the following concentration terms is/are independent of temperature ?
View SolutionWhich of the following is independent of Temperature ?
View Solution
