Which of the following is not possible ?
n=3,l=2,m=0
n=1,l=0,m=0
n=3,l=3,m=2
n=4,l=3,m=−3
The correct Answer is:C
To determine which set of quantum numbers is not possible, we need to analyze each set based on the rules governing quantum numbers. Quantum numbers include the principal quantum number (N), azimuthal quantum number (L), and magnetic quantum number (M).
1. Understanding Quantum Numbers:
- Principal Quantum Number (N): Indicates the main energy level or shell. It can take positive integer values (1, 2, 3, ...).
- Azimuthal Quantum Number (L): Indicates the subshell and can take values from 0 to (N-1).
- L = 0 corresponds to s subshell
- L = 1 corresponds to p subshell
- L = 2 corresponds to d subshell
- L = 3 corresponds to f subshell
- Magnetic Quantum Number (M): Indicates the orientation of the orbital and can take values from -L to +L, including 0.
2. Analyzing Each Set of Quantum Numbers:
- Option A: N = 3, L = 2, M = 0
- N = 3 allows L values of 0, 1, 2 (valid).
- L = 2 corresponds to a d subshell, and M can be -2, -1, 0, +1, +2 (valid).
- Conclusion: This set is possible.
- Option B: N = 1, L = 0, M = 0
- N = 1 allows L values of 0 (valid).
- L = 0 corresponds to an s subshell, and M can only be 0 (valid).
- Conclusion: This set is possible.
- Option C: N = 3, L = 3, M = 2
- N = 3 allows L values of 0, 1, 2 (invalid because L cannot equal N).
- Conclusion: This set is not possible.
- Option D: N = 4, L = 3, M = -3
- N = 4 allows L values of 0, 1, 2, 3 (valid).
- L = 3 corresponds to an f subshell, and M can be -3, -2, -1, 0, +1, +2, +3 (valid).
- Conclusion: This set is possible.
3. Final Conclusion:
- The only set of quantum numbers that is not possible is Option C: N = 3, L = 3, M = 2.
Summary:
The answer to the question "Which of the following is not possible?" is Option C.
- Principal Quantum Number (N): Indicates the main energy level or shell. It can take positive integer values (1, 2, 3, ...).
- Azimuthal Quantum Number (L): Indicates the subshell and can take values from 0 to (N-1).
- L = 0 corresponds to s subshell
- L = 1 corresponds to p subshell
- L = 2 corresponds to d subshell
- L = 3 corresponds to f subshell
- Magnetic Quantum Number (M): Indicates the orientation of the orbital and can take values from -L to +L, including 0.
- Option A: N = 3, L = 2, M = 0
- N = 3 allows L values of 0, 1, 2 (valid).
- L = 2 corresponds to a d subshell, and M can be -2, -1, 0, +1, +2 (valid).
- Conclusion: This set is possible.
- Option B: N = 1, L = 0, M = 0
- N = 1 allows L values of 0 (valid).
- L = 0 corresponds to an s subshell, and M can only be 0 (valid).
- Conclusion: This set is possible.
- N = 3 allows L values of 0, 1, 2 (invalid because L cannot equal N).
- Conclusion: This set is not possible.
- N = 4 allows L values of 0, 1, 2, 3 (valid).
- L = 3 corresponds to an f subshell, and M can be -3, -2, -1, 0, +1, +2, +3 (valid).
- Conclusion: This set is possible.
- The only set of quantum numbers that is not possible is Option C: N = 3, L = 3, M = 2.
The answer to the question "Which of the following is not possible?" is Option C.
Topper's Solved these Questions
- ATOMIC STRUCTURE BOOK - MTG IIT JEE FOUNDATIONCHAPTER - ATOMIC STRUCTURE EXERCISE - Exercise (Match the following)
5 Videos
- ATOMIC STRUCTURE BOOK - MTG IIT JEE FOUNDATIONCHAPTER - ATOMIC STRUCTURE EXERCISE - Exercise (Assertion & Reason Type)
10 Videos
- ATOMIC STRUCTURE BOOK - MTG IIT JEE FOUNDATIONCHAPTER - ATOMIC STRUCTURE EXERCISE - Solved Examples
11 Videos
- ACIDS , BASES AND SALTS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - ACIDS , BASES AND SALTS EXERCISE - Olympiad /HOTS Corner
20 Videos
- CARBON AND ITS COMPOUNDS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - CARBON AND ITS COMPOUNDS EXERCISE - Olympaid/HOTS Corner
25 Videos
Similar Questions
List-I List-II(P) Aufbau principle(1)Δx×Δp≈h/4π(Q) de-Broglie(2) Orientation of electron in an orbital(R) Hund'srule(3) Electronic configuration(S) Heisenberg principle(4)λ=h/mv
View SolutionAssertion : An orbital cannot have more than two electrons.
Reason : The two electrons in an orbital have opposite spin.
View SolutionHow many electrons can be filled in all the orbitals with n+l=5 ?
View SolutionWhat of the following options shows the correct arrangement of different substances with increasing pH values ?
View SolutionAn organic compound 'A' on heating with concentrated H2SO4 forms a compound 'B' which on addition of one mole of hydrogen in presence of Ni forms a compound 'C'. One mole of compound 'C' on combustion forms two moles of CO2 and three moles of H2O.
Which of the following represents the compound 'C' ?
View SolutionWhich of the following compound is possible :
View Solution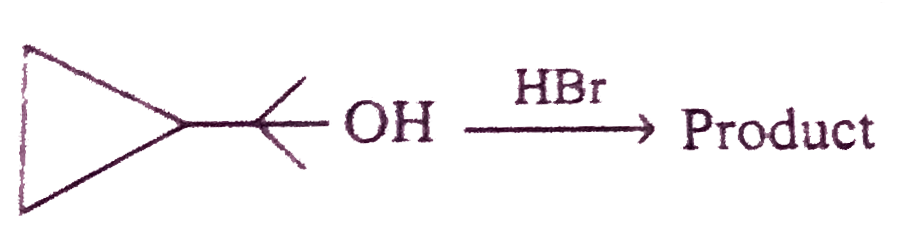
Which of the following are possible are possible products in significant amounts?
View Solution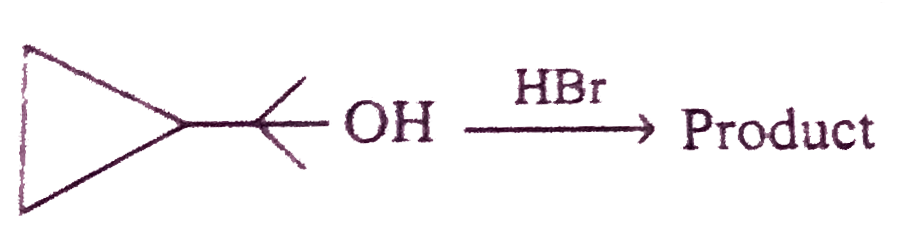
Which of the following are possible are possible products in significant amounts?
View SolutionWhich of the following is not possible ?
View SolutionWhich of the following orbitals are not possible ?
2s, 2p, 3f, 3d.
View SolutionWhich of the following oxocation is not possible
View SolutionWhich of the following is not possible
View SolutionWhich one of the following is possible?
View Solutionनिम्न में से कौनसा सम्भव नहीं है
View Solutionनिम्न में कौनसा कक्षक असम्भव है
View SolutionWhich of the following is not possible?
View Solution
