What is indicated by:
Blue flame of a bunsen burner?
Step-by-Step Solution:
1. Understanding the Bunsen Burner: A Bunsen burner is a common laboratory device used for heating, sterilization, and combustion. It produces a flame when gas is ignited.
2. Identifying the Flame Color: When the Bunsen burner is lit, it can produce different flame colors depending on the combustion process. The two main flame colors are blue and yellow.
3. Complete vs. Incomplete Combustion:
- Complete Combustion: This occurs when there is enough oxygen for the fuel to react completely. In this case, hydrocarbons burn efficiently, producing carbon dioxide and water. The flame appears blue.
- Incomplete Combustion: This occurs when there is insufficient oxygen, leading to the production of carbon monoxide and soot. The flame appears yellow.
4. Conclusion: The blue flame of a Bunsen burner indicates that complete combustion of the fuel (usually a hydrocarbon) is taking place. This is characterized by a higher temperature and a cleaner burn.
Final Answer: The blue flame of a Bunsen burner indicates complete combustion of hydrocarbons.
---
- Complete Combustion: This occurs when there is enough oxygen for the fuel to react completely. In this case, hydrocarbons burn efficiently, producing carbon dioxide and water. The flame appears blue.
- Incomplete Combustion: This occurs when there is insufficient oxygen, leading to the production of carbon monoxide and soot. The flame appears yellow.
Topper's Solved these Questions
- FOOTSTEPS TOWARDS CBSE BOARDBOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS CBSE BOARDEXERCISE - SECTION - C
39 Videos
- FOOTSTEPS TOWARDS CBSE BOARDBOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS CBSE BOARDEXERCISE - SECTION - C
39 Videos
- FOOTSTEPS TOWARDS (JEE MAIN) BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS (JEE MAIN) EXERCISE - Integer/Numerical Value Type
5 Videos
- METALS AND NON METALS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - METALS AND NON METALS EXERCISE - Olympiad/HOTS Corner
18 Videos
Similar Questions
The members of alkene family are represented by the general formula CnH2n. Now answer the following:
What do n and 2n signify?
View SolutionThe members of alkene family are represented by the general formula CnH2n. Now answer the following:
What do n and 2n signify?
View SolutionFrom the given list, how many pairs of elements form ionic bonds when they react?
Bromine, Carbon, Iron, Sodium, Sulphur
View SolutionMetals are extracted from their ores by different methods depending on their position in the activity series
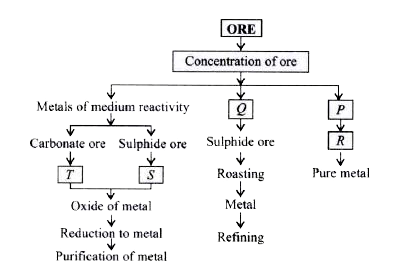
Match the Column-I with Column-II and select the correct option from the codes given below.
Column-I Column-II(A) Highly reactive metals(i) S (B) Calcination(ii) T (C) Electrolytic reduction(iii) P(D) Less reactive metal(iv) Q(E) Roasting(V) Q
View SolutionWhich letter in the diagram depicts the hottest portion of a Bunsen burner flame?
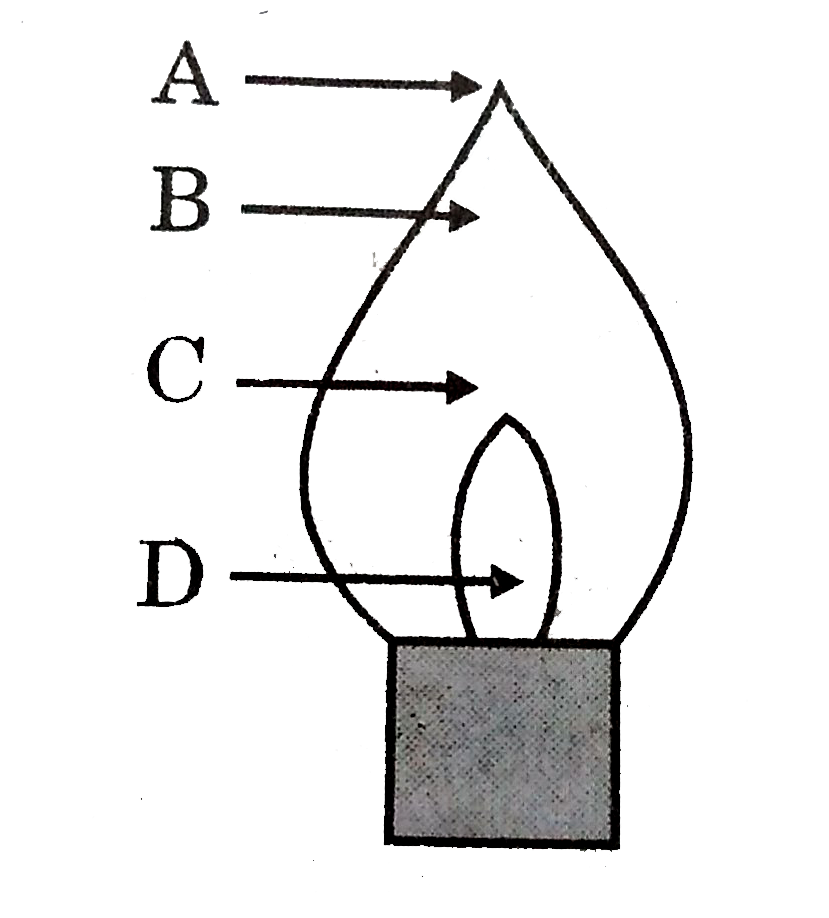
View SolutionWhich of the following imparts violet colouration to the non-luminous flame of Bunsen burner ?
View SolutionWhich of the following imparts violet colouration to the Bunsen burner nonluminous flame ?
View SolutionThe hottest part of the flame of a Bunsen burner is the
View SolutionThe elements of group IA provide a colour to the flame of Bunsen burner due to
View Solutionसमूह IA के तत्व बुन्सन बर्नर की ज्वाला को रंग किस कारण से प्रदान करते है?
View SolutionWhy do alkali metals impart characteristic colour to the flame of bunsen burner?
View SolutionWhich alkali metal chloride imparts violet colour to the bunsen burner flame ?
View SolutionMatch the flame colours of the alkali and alkaline earth metal salts in the bunsen burner
View SolutionMatch the flame colours of the alkali and alkaline earth metal salts in the bunsen burner

View SolutionWhat is the colour of the flame on heating potassium in the flame of a Bunsen burner?
View Solution(a) How does the supply of air affect combustion of saturated hydrocarbons?
(b) What is indicated by:
(i) sooty flame (ii) blue flame of a bunsen burner?
(c ) Why are holes provided at the bottom of a bunsen burner?
View Solution
