Assertion : Dry cell is used to convert chemical energy into electrical energy .
Reason : Chemical reactions occur in the electrolyte of the dry cell that generate electricity .
If both assertion and reason are true and reason is the correct explanation of assertion .
If both assertion and reason are true but reason is not the correct explanation of assertion .
If assertion is true reason is false .
If assertion is false but reason is true .
The correct Answer is:A
Step-by-Step Solution:
1. Understanding the Assertion:
- The assertion states that a dry cell is used to convert chemical energy into electrical energy.
- This is true because dry cells are designed specifically to store chemical energy and convert it into electrical energy when needed.
2. Understanding the Reason:
- The reason provided states that chemical reactions occur in the electrolyte of the dry cell, which generates electricity.
- This is also true. In a dry cell, there is an electrolyte that facilitates chemical reactions. These reactions are responsible for the conversion of stored chemical energy into electrical energy.
3. Evaluating the Relationship:
- Since both the assertion and the reason are true, we need to determine if the reason correctly explains the assertion.
- The reason explains how the dry cell converts chemical energy into electrical energy through chemical reactions in the electrolyte.
4. Conclusion:
- Both the assertion and the reason are true, and the reason is indeed the correct explanation of the assertion. Therefore, the correct answer is that both statements are true, and the reason is the correct explanation of the assertion.
Final Answer:
Both the assertion and the reason are true, and the reason is the correct explanation of the assertion.
---
- The assertion states that a dry cell is used to convert chemical energy into electrical energy.
- This is true because dry cells are designed specifically to store chemical energy and convert it into electrical energy when needed.
- The reason provided states that chemical reactions occur in the electrolyte of the dry cell, which generates electricity.
- This is also true. In a dry cell, there is an electrolyte that facilitates chemical reactions. These reactions are responsible for the conversion of stored chemical energy into electrical energy.
- Since both the assertion and the reason are true, we need to determine if the reason correctly explains the assertion.
- The reason explains how the dry cell converts chemical energy into electrical energy through chemical reactions in the electrolyte.
- Both the assertion and the reason are true, and the reason is indeed the correct explanation of the assertion. Therefore, the correct answer is that both statements are true, and the reason is the correct explanation of the assertion.
Both the assertion and the reason are true, and the reason is the correct explanation of the assertion.
Topper's Solved these Questions
- ELECTRICITY AND CIRCUITS Book:MTG IIT JEE FOUNDATIONChapter:ELECTRICITY AND CIRCUITS Exercise:EXERCISE (Comprehension Type)
Explore 7 Videos
- ELECTRICITY AND CIRCUITS Book:MTG IIT JEE FOUNDATIONChapter:ELECTRICITY AND CIRCUITS Exercise:EXERCISE (Subjective Problems (Very Short Answer Type)
Explore 10 Videos
- ELECTRICITY AND CIRCUITS Book:MTG IIT JEE FOUNDATIONChapter:ELECTRICITY AND CIRCUITS Exercise:EXERCISE (Match the Following)
Explore 3 Videos
- FUN WITH MAGNETSBook:MTG IIT JEE FOUNDATIONChapter:FUN WITH MAGNETSExercise:Olympaid/HOTS Corner
Explore 8 Videos
Similar Questions
There is a variety of materials that are used to make components of electrical circuits . These materials have good electrical conductivity . Ispite of having the largest conducivity among metals , silver is not used to make electric wires . Aluminium copper and are generally used to make electic wires .Not only conductors , insulators are alos important .the electrical wires are covered with plastic and rubber like materials . This is used to prevent short circuit and also to protect the user .
What kind of materials are used to make electrical wires in an electric circuit ?
View SolutionThe given figure is made up of two parallel lines . One is thin and long other is short and thick .What does this mean ?
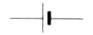
View SolutionList -I and List -II are given as options (a) ,(b) , (c ) and (d) out of which one is correct .

View SolutionWhich figure correctly shows two iron nails hanging from a bar magnet?
View SolutionIn which cell the free energy of a chemical reaction is directly converted into electricity ?
View SolutionAssertion:Electrolytic cell uses electrical energy to carry non-spontaneous chemical reactions. Reason : Chemical energy of a spontaneous redox reaction can be converted into electrical energy.
View Solutionविद्युत-अपघटनी सेल में रासायनिक ऊर्जा को विद्युत ऊर्जा में परिवर्तित किया जाता है।
View SolutionWhich cell converts electrical energy into chemical energy ?
View Solution……………. Is an electrochemical cell which converts electrical energy into chemical change (Reaction).
View SolutionConsider the following statements : (i) In Galvanic cell, chemical energy is converted into electrical energy. (ii) In electrolytic cell, electrical energy is converted into chemical energy. (iii) In Voltaic cell, electrical energy is converted into chemical energy. Which of the above statement is/are not correct ?
View SolutionAssertion (A) : Batteries and fuel cells convert chemical energy into electrical energy. Reason (R) : The reactions carried out electrochemically are not energy efficient and more polluting.
View SolutionAssertion: An electrolytic cell uses electrical energy to carry out inert chemical reactions. Rationale: The chemical energy of an automatic redox reaction can be converted into electrical energy.
View Solutionकिस सेल से रासायनिक अभिकिया की मुक्त ऊर्जा सीधे ही विधुत में परिवर्तित होती है ?
View SolutionAssertion:Electrolytic cell uses electrical energy to carry non-spontaneous chemical reactions. Reason : Chemical energy of a spontaneous redox reaction can be converted into electrical energy.
View SolutionIn which cell, electrical energy is converted into chemical energy:
View Solution