There are two substances A and B The mass of substance B is found tobe 3 times the mass of substance A, but the volume of substance A is 1/9 th the volume of substance B. Which of the two substances possesses greater density?
To determine which of the two substances, A or B, possesses greater density, we can follow these steps:
Step 1: Define the relationship between the masses of substances A and B.
According to the problem, the mass of substance B (M_B) is 3 times the mass of substance A (M_A). We can express this mathematically as:
MB=3×MA
Step 2: Define the relationship between the volumes of substances A and B.
The problem states that the volume of substance A (V_A) is 1/9th of the volume of substance B (V_B). This can be expressed as:
VA=19×VB
From this, we can also express V_B in terms of V_A:
VB=9×VA
Step 3: Write the formula for density.
Density (D) is defined as mass divided by volume. Therefore, we can write the densities of substances A and B as:
DA=MAVA
DB=MBVB
Step 4: Substitute the values of mass and volume into the density formulas.
Now, we can substitute the expressions we derived for M_B and V_B into the density formula for substance B:
DB=MBVB=3×MA9×VA
Step 5: Simplify the expression for D_B.
Now, we simplify the expression for D_B:
DB=3×MA9×VA=13×MAVA
Since MAVA is the density of substance A (D_A), we can write:
DB=13×DA
Step 6: Compare the densities.
From the relationship we derived:
DB=13×DA
This shows that the density of substance A is three times that of substance B:
DA=3×DB
Conclusion:
Since DA is greater than DB, we conclude that substance A possesses greater density than substance B.
According to the problem, the mass of substance B (M_B) is 3 times the mass of substance A (M_A). We can express this mathematically as:
MB=3×MA
The problem states that the volume of substance A (V_A) is 1/9th of the volume of substance B (V_B). This can be expressed as:
VA=19×VB
From this, we can also express V_B in terms of V_A:
VB=9×VA
Density (D) is defined as mass divided by volume. Therefore, we can write the densities of substances A and B as:
DA=MAVA
DB=MBVB
Now, we can substitute the expressions we derived for M_B and V_B into the density formula for substance B:
DB=MBVB=3×MA9×VA
Now, we simplify the expression for D_B:
DB=3×MA9×VA=13×MAVA
Since MAVA is the density of substance A (D_A), we can write:
DB=13×DA
From the relationship we derived:
DB=13×DA
This shows that the density of substance A is three times that of substance B:
DA=3×DB
Since DA is greater than DB, we conclude that substance A possesses greater density than substance B.
Similar Questions
Knowledge Check
All organic substances possess
ACarbon, Hydrogen and OxygenBCarbon, Oxygen and NitrogenCCarbon and hydrogenDCarbon, Hydrogen, Oxygen and Nitrogen.The density of a substance is defined as:
Adensity =volume/massBdensity=volume/(mass)2Cdensity=mass/volumeDdensity=(volume)2/mass.Which one of the following substances possesses the highest elasticity-
ArubberBglassCsteelDcopper
The vapour density of a substance is 24. What is the gram molecular weight of the substance?
View SolutionHeat of conversion of substance A to substance B is equal to (kJ)
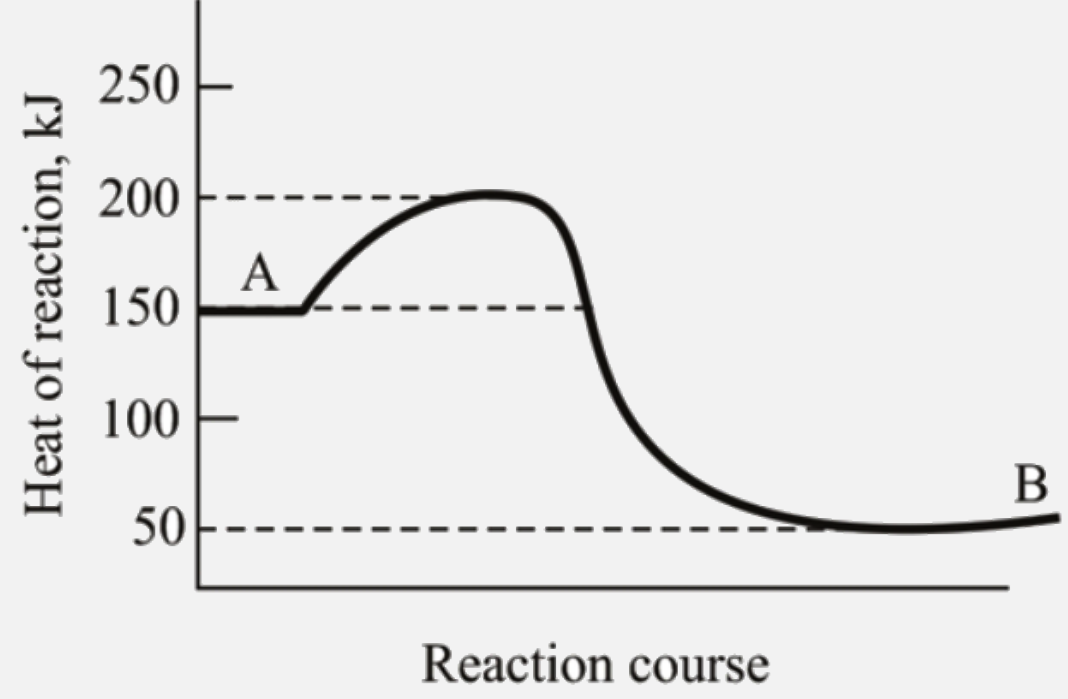
View SolutionThe substances A and B when brought together form a substance C with the evolution of heat. The substance C is
View SolutionAntiferromagnetic substance possess:
View Solution1 gm equivalent of a substance is the weight of the substance which is equivalent to
View Solution
